Abstract
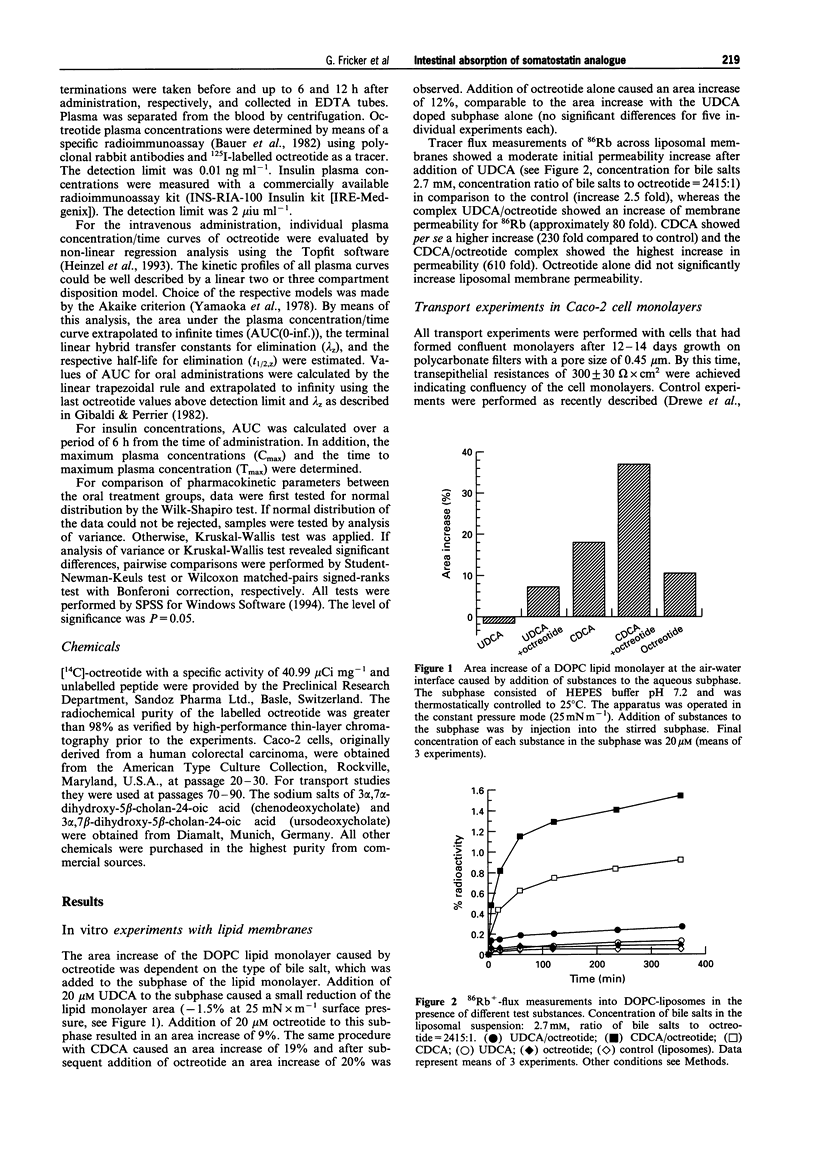
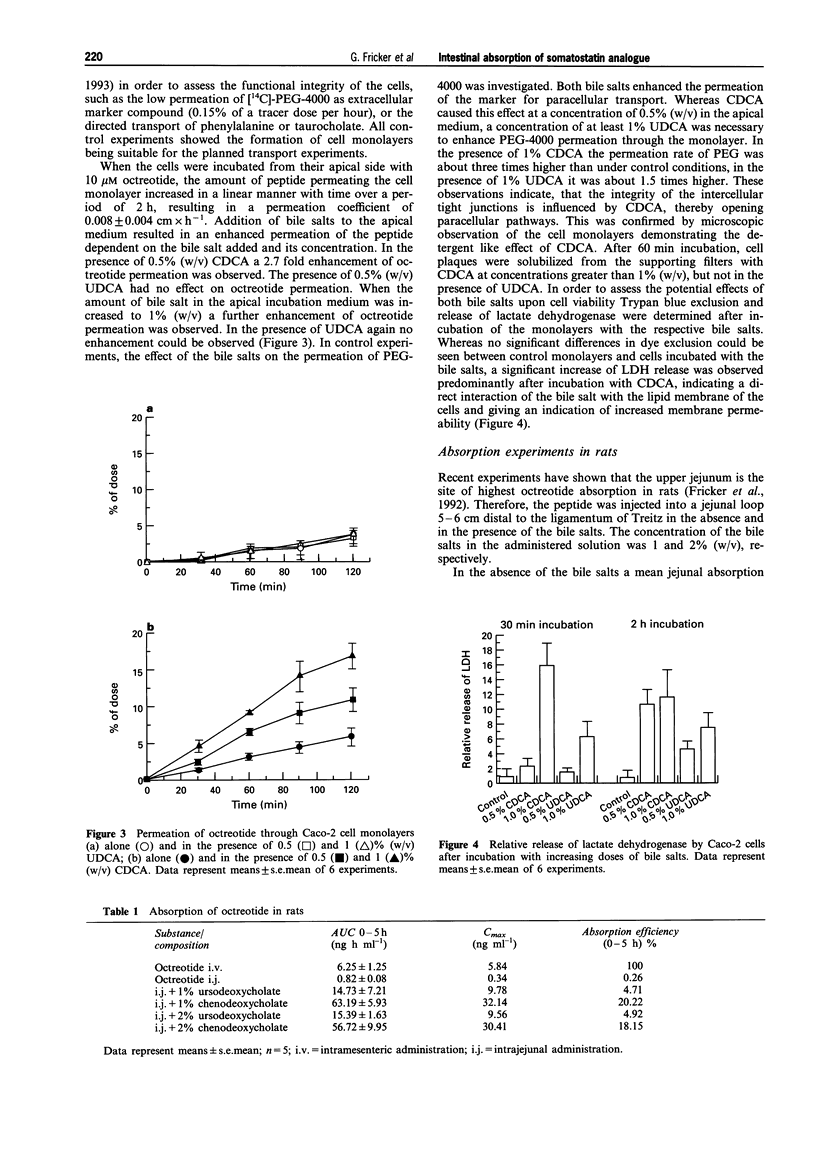
1. The potential of bile salts to improve the enteral absorption of octreotide, an orally active somatostatin analogue, was investigated by a combination of in vitro, in situ and in vivo experiments. 2. Incorporation of octreotide into lipid monolayers (as measured by area increase of the monolayer at constant surface pressure using a Langmuir-Blodgett trough set-up) depended on the type of bile salt used for monolayer pre-treatment. Addition of 20 microM octreotide to the subphase containing 20 microM of the dihydroxylated bile salt ursodeoxycholate (UDCA) causes a 9% increase in area, whereas addition of octreotide to the subphase containing the 7 alpha-enantiomer of UDCA, chenodeoxycholate (CDCA), resulted in an area increase of the lipid monolayer of 20%. Area increase by octreotide alone was not significantly different from the increase of octreotide and UDCA in combination. 3. CDCA and UDCA in combination with octreotide increased the permeability of liposomal membranes for rubidium ions, whereas octreotide alone did not significantly change the permeability. This indicates membrane distortion as a possible cause for the enhanced absorption of octreotide by bile salts. 4. In polarized Caco-2 cell monolayers octreotide exhibited a permeation coefficient of 0.008 +/- 0.004 cm h-1. Addition of 0.2-1% of UDCA to the apical incubation medium had no significant effect upon the permeation coefficient. In contrast, 0.2-1% CDCA in the incubation medium resulted in a significant increase (P < 0.05) of the monolayer permeability of octreotide (0.015-0.037 cm h-1). 5. Octreotide was absorbed as the intact peptide from the gastrointestinal tract in rats with an absorption efficiency of 0.26%. Coadministration of bile salt resulted in a dose-dependent increase in absorption efficiency of the peptide up to 20.2%. The observed effect was more pronounced for CDCA than for UDCA. 6. The effect of CDCA and UDCA on octreotide absorption in vivo was assessed in a pharmacokinetic study with healthy volunteers. After oral administration of 4 mg octreotide in the presence of 100 mg bile salt, an average bioavailability of the peptide of 1.26% was achieved in the presence of CDCA, whereas in the presence of UDCA a bioavailability of only 0.13% was reached. This difference was statistically significant (P < 0.01). 7. In conclusion, the co-administration of CDCA is able to enhance the enteral absorption of octreotide. The in vitro and in situ experiments were predictive for the observed effect in human subjects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderberg E. K., Nyström C., Artursson P. Epithelial transport of drugs in cell culture. VII: Effects of pharmaceutical surfactant excipients and bile acids on transepithelial permeability in monolayers of human intestinal epithelial (Caco-2) cells. J Pharm Sci. 1992 Sep;81(9):879–887. doi: 10.1002/jps.2600810908. [DOI] [PubMed] [Google Scholar]
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Drewe J., Fricker G., Vonderscher J., Beglinger C. Enteral absorption of octreotide: absorption enhancement by polyoxyethylene-24-cholesterol ether. Br J Pharmacol. 1993 Feb;108(2):298–303. doi: 10.1111/j.1476-5381.1993.tb12799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahr A., Nimmerfall F., Wenger R. Interactions of cyclosporine and some derivatives with model membranes: binding and ion permeability changes. Transplant Proc. 1994 Oct;26(5):2837–2841. [PubMed] [Google Scholar]
- Fricker G., Bruns C., Munzer J., Briner U., Albert R., Kissel T., Vonderscher J. Intestinal absorption of the octapeptide SMS 201-995 visualized by fluorescence derivatization. Gastroenterology. 1991 Jun;100(6):1544–1552. doi: 10.1016/0016-5085(91)90651-z. [DOI] [PubMed] [Google Scholar]
- Fricker G., Drewe J., Vonderscher J., Kissel T., Beglinger C. Enteral absorption of octreotide. Br J Pharmacol. 1992 Apr;105(4):783–786. doi: 10.1111/j.1476-5381.1992.tb09057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuessl H. S., Domin J., Bloom S. R. Oral absorption of the somatostatin analogue SMS 201-995: theoretical and practical implications. Clin Sci (Lond) 1987 Feb;72(2):255–257. doi: 10.1042/cs0720255. [DOI] [PubMed] [Google Scholar]
- Gasco M. R., Trotta M., Eandi M. The influence of bile salts on the absorption in vitro and in vivo of propranolol. J Pharm Biomed Anal. 1984;2(3-4):425–439. doi: 10.1016/0731-7085(84)80046-1. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Borchardt R. T. Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta. 1990 Jul 20;1035(1):97–103. doi: 10.1016/0304-4165(90)90179-z. [DOI] [PubMed] [Google Scholar]
- Kissel T., Drewe J., Bantle S., Rummelt A., Beglinger C. Tolerability and absorption enhancement of intranasally administered octreotide by sodium taurodihydrofusidate in healthy subjects. Pharm Res. 1992 Jan;9(1):52–57. doi: 10.1023/a:1018927710280. [DOI] [PubMed] [Google Scholar]
- Köhler E., Duberow-Drewe M., Drewe J., Ribes G., Loubatiéres-Mariani M. M., Mazer N., Gyr K., Beglinger C. Absorption of an aqueous solution of a new synthetic somatostatin analogue administered to man by gavage. Eur J Clin Pharmacol. 1987;33(2):167–171. doi: 10.1007/BF00544562. [DOI] [PubMed] [Google Scholar]
- Lee V. H., Yamamoto A., Kompella U. B. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Crit Rev Ther Drug Carrier Syst. 1991;8(2):91–192. [PubMed] [Google Scholar]
- Murakami T., Sasaki Y., Yamajo R., Yata N. Effect of bile salts on the rectal absorption of sodium ampicillin in rats. Chem Pharm Bull (Tokyo) 1984 May;32(5):1948–1955. doi: 10.1248/cpb.32.1948. [DOI] [PubMed] [Google Scholar]
- Pless J., Bauer W., Briner U., Doepfner W., Marbach P., Maurer R., Petcher T. J., Reubi J. C., Vonderscher J. Chemistry and pharmacology of SMS 201-995, a long-acting octapeptide analogue of somatostatin. Scand J Gastroenterol Suppl. 1986;119:54–64. doi: 10.3109/00365528609087432. [DOI] [PubMed] [Google Scholar]
- Poelma F. G., Tukker J. J., Crommelin D. J. Intestinal absorption of drugs. I: The influence of taurocholate on the absorption of dantrolene in the small intestine of the rat. J Pharm Sci. 1989 Apr;78(4):285–289. doi: 10.1002/jps.2600780405. [DOI] [PubMed] [Google Scholar]
- Roda A., Minutello A., Angellotti M. A., Fini A. Bile acid structure-activity relationship: evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J Lipid Res. 1990 Aug;31(8):1433–1443. [PubMed] [Google Scholar]
- Tengamnuay P., Mitra A. K. Bile salt-fatty acid mixed micelles as nasal absorption promoters of peptides. II. In vivo nasal absorption of insulin in rats and effects of mixed micelles on the morphological integrity of the nasal mucosa. Pharm Res. 1990 Apr;7(4):370–375. doi: 10.1023/a:1015867305641. [DOI] [PubMed] [Google Scholar]
- Vadnere M., Lindenbaum S. Distribution of bile salts between 1-octanol and aqueous buffer. J Pharm Sci. 1982 Aug;71(8):875–881. doi: 10.1002/jps.2600710809. [DOI] [PubMed] [Google Scholar]
- Williams G., Ball J. A., Burrin J. M., Joplin G. F., Bloom S. R. Effective and lasting growth-hormone suppression in active acromegaly with oral administration of somatostatin analogue SMS 201-995. Lancet. 1986 Oct 4;2(8510):774–778. doi: 10.1016/s0140-6736(86)90300-4. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Treanor L. L. Characterization of bile acid binding to rat intestinal brush border membranes. J Membr Biol. 1977 May 12;33(3-4):213–230. doi: 10.1007/BF01869517. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Ikeda C., Sekine Y. Intestinal absorption of a beta-adrenergic blocking agents nadolol. II. Mechanism of the inhibitory effect on the intestinal absorption of nadolol by sodium cholate in rats. Chem Pharm Bull (Tokyo) 1986 Sep;34(9):3836–3843. doi: 10.1248/cpb.34.3836. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- van Hoogdalem E. J., Heijligers-Feijen C. D., Mathôt R. A., Wackwitz A. T., van Bree J. B., Verhoef J. C., de Boer A. G., Breimer D. D. Rectal absorption enhancement of cefoxitin and desglycinamide arginine vasopressin by sodium tauro-24,25-dihydrofusidate in conscious rats. J Pharmacol Exp Ther. 1989 Nov;251(2):741–744. [PubMed] [Google Scholar]
- van Hoogdalem E. J., Heijligers-Feijen C. D., Verhoef J. C., de Boer A. G., Breimer D. D. Absorption enhancement of rectally infused insulin by sodium tauro-24,25-dihydrofusidate (STDHF) in rats. Pharm Res. 1990 Feb;7(2):180–183. doi: 10.1023/a:1015837004307. [DOI] [PubMed] [Google Scholar]



