Abstract
1. Recent work in our laboratory has demonstrated that intrathecal administration of a selective antagonist of calcitonin gene-related peptide (CGRP), CGRP8-37, increased the hindpaw withdrawal latency (HWL) to thermal stimulation and hindpaw withdrawal threshold (HWT) to pressure in normal rats, and that these effects were more pronounced than in rats with mononeuropathy. 2. The present study was performed to investigate the effects of intrathecal administration of CGRP8-37 on the HWL and HWT in rats with unilateral hindpaw inflammation induced by subcutaneous injection of carrageenin. The effect of naloxone was also studied. 3. Subcutaneous injection of 0.1 ml of carrageenin into the plantar region of the left hindpaw induced a significant increase in the volume of the ipsilateral hindpaw (P < 0.001), and significant bilateral decreases of the HWL to thermal stimulation (ipsilateral: P < 0.001; contralateral: P < 0.01) and HWT to pressure (ipsilateral: P < 0.001; contralateral: P < 0.01). 4. Intrathecal administration of 10 nmol of CGRP8-37, but not of 1 or 5 nmol, induced a significant bilateral increase in the HWL and HWT in rats with experimentally induced inflammation (thermal test: P < 0.001; mechanical test: P < 0.001). 5. The effect of intrathecal administration of 10 nmol CGRP8-37 on HWL and HWT was significantly more pronounced in intact rats than in rats with experimentally induced inflammation (ipsilateral: P < 0.001; contralateral: P < 0.001). 6. The effect of CGRP8-37 on withdrawal responses in the inflamed paw was partly reversed by intrathecal injection of naloxone at a dose of 88 nmol in the thermal (ipsilateral: P < 0.01; contralateral: P = 0.14) and mechanical tests (ipsilateral: P < 0.05; contralateral: P = 0.60). 7. A significant bilateral increase in the concentration of CGRP-like immunoreactivity in the perfusate of both hindpaws was demonstrated 24 h after unilateral injection of carrageenin (ipsilateral: P < 0.001; contralateral: P < 0.05). There was also an increase in the amount of CGRP-like immunoreactivity in the cerebrospinal fluid (P < 0.001), but not in plasma (P = 0.75). 8. The present study demonstrates that acute experimentally-induced unilateral hindpaw inflammation, induces bilateral increases in the amount of CGRP-like immunoreactivity in hindpaw perfusates. Intrathecal administration of CGRP8-37 increased the HWL to thermal stimulation and HWT to pressure bilaterally. 9. The results indicate that CGRP plays a role in the transmission of presumed nociceptive information in the spinal cord of rats with experimentally induced inflammation. Furthermore, our findings suggest that opioids can modulate CGRP-related effects in the spinal cord.
Full text
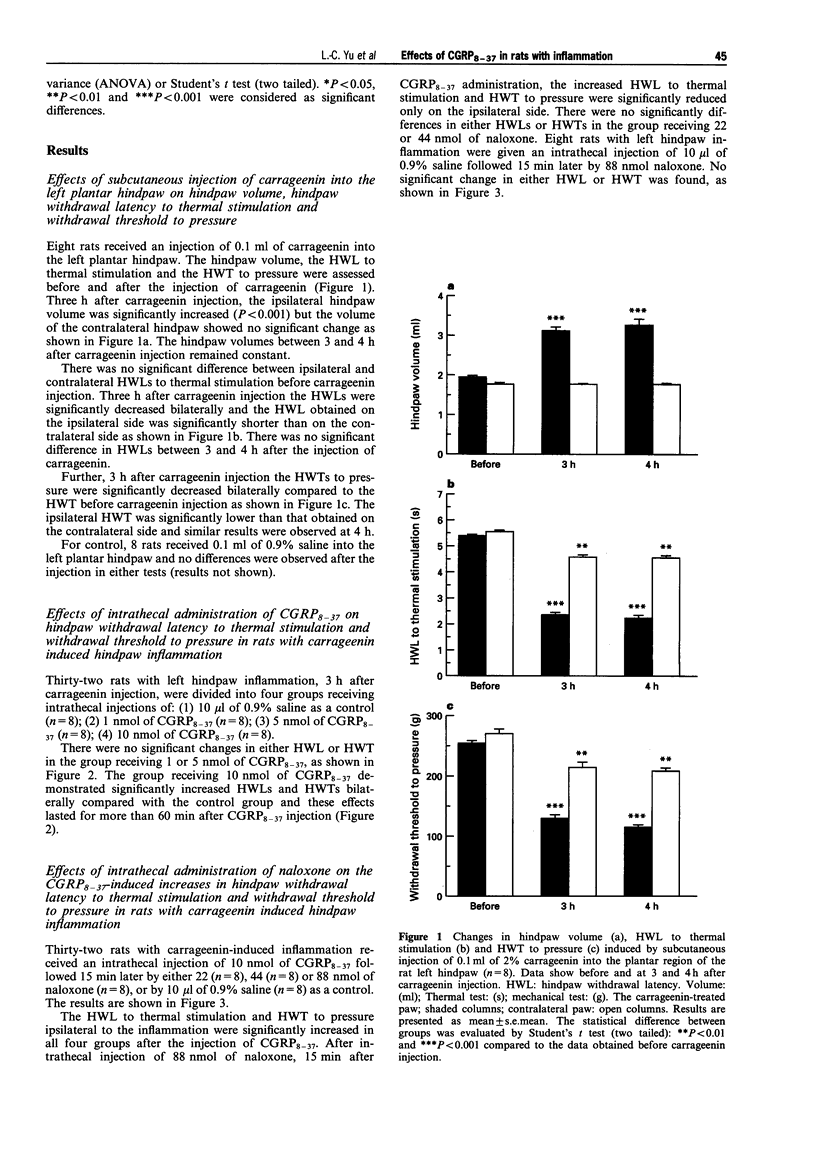
PDF

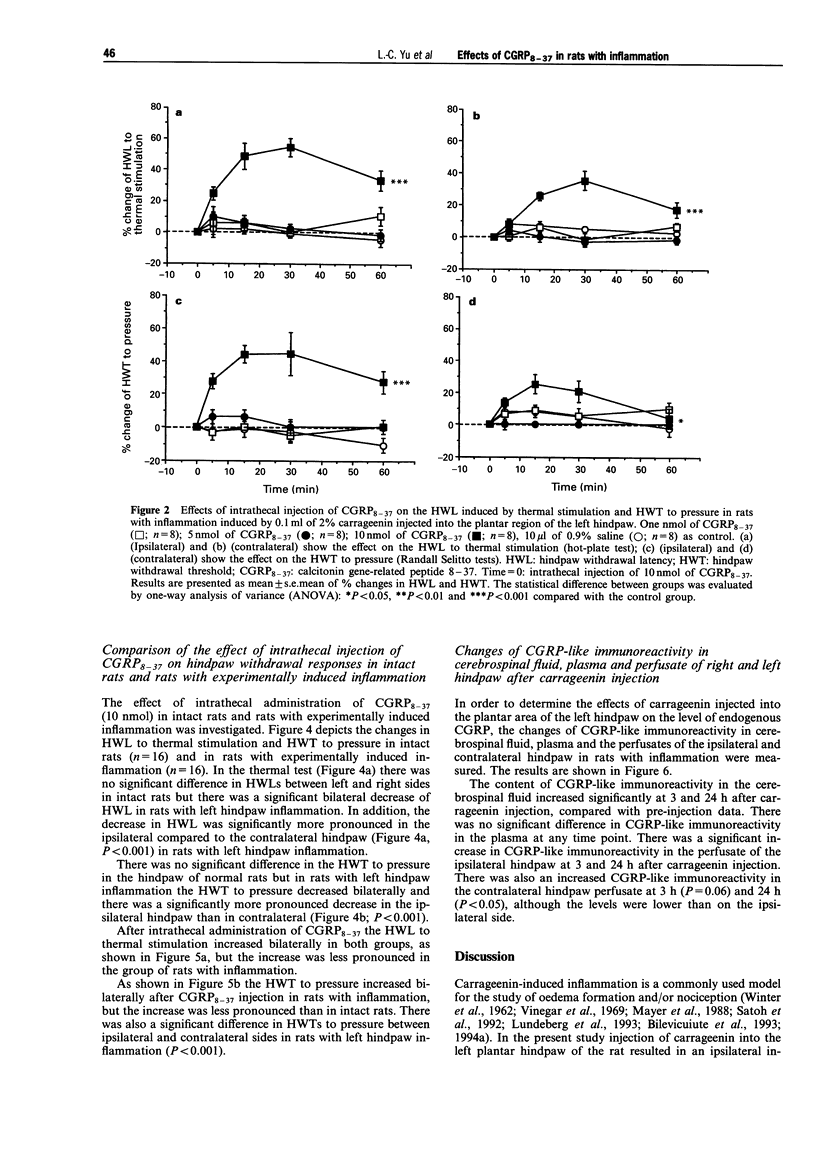



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
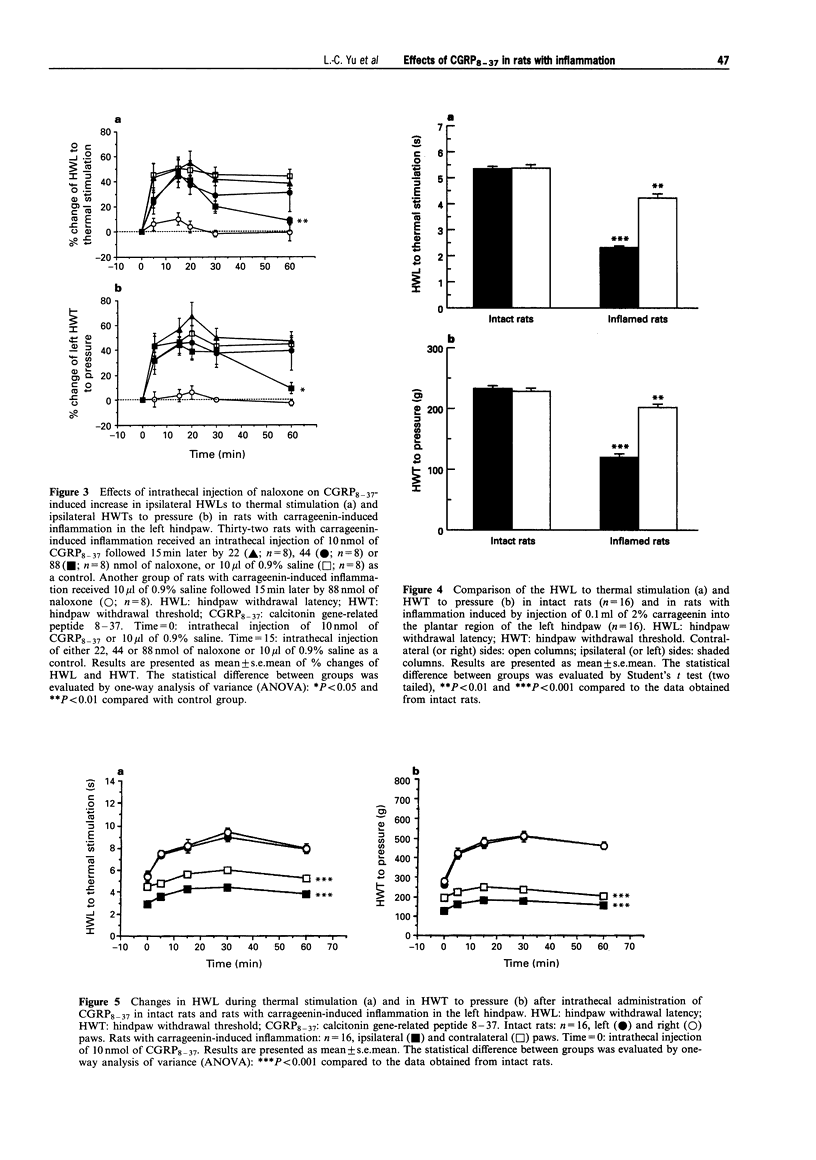
- Biella G., Panara C., Pecile A., Sotgiu M. L. Facilitatory role of calcitonin gene-related peptide (CGRP) on excitation induced by substance P (SP) and noxious stimuli in rat spinal dorsal horn neurons. An iontophoretic study in vivo. Brain Res. 1991 Sep 20;559(2):352–356. doi: 10.1016/0006-8993(91)90024-p. [DOI] [PubMed] [Google Scholar]
- Bileviciute I., Lundeberg T., Ekblom A., Theodorsson E. Bilateral changes of substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity in rat knee joint synovial fluid during acute monoarthritis. Neurosci Lett. 1993 Apr 16;153(1):37–40. doi: 10.1016/0304-3940(93)90071-r. [DOI] [PubMed] [Google Scholar]
- Bileviciute I., Lundeberg T., Ekblom A., Theodorsson E. Substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity (-LI) in rat knee joint synovial fluid during acute monoarthritis is not correlated with concentrations of neuropeptide-LI in cerebrospinal fluid and plasma. Neurosci Lett. 1994 Feb 14;167(1-2):145–148. doi: 10.1016/0304-3940(94)91048-0. [DOI] [PubMed] [Google Scholar]
- Bileviciute I., Lundeberg T., Ekblom A., Theodorsson E. The effect of a single intraperitoneal dose of hrIL-1 alpha on substance P-, neurokinin A-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity in cerebrospinal fluid, plasma and knee joint synovial fluid in the rat. Regul Pept. 1994 Aug 31;53(1):71–76. doi: 10.1016/0167-0115(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Cameron A. A., Leah J. D., Snow P. J. The coexistence of neuropeptides in feline sensory neurons. Neuroscience. 1988 Dec;27(3):969–979. doi: 10.1016/0306-4522(88)90200-x. [DOI] [PubMed] [Google Scholar]
- Chiba T., Yamaguchi A., Yamatani T., Nakamura A., Morishita T., Inui T., Fukase M., Noda T., Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol. 1989 Feb;256(2 Pt 1):E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Collin E., Frechilla D., Pohl M., Bourgoin S., Le Bars D., Hamon M., Cesselin F. Opioid control of the release of calcitonin gene-related peptide-like material from the rat spinal cord in vivo. Brain Res. 1993 Apr 23;609(1-2):211–222. doi: 10.1016/0006-8993(93)90875-n. [DOI] [PubMed] [Google Scholar]
- Cridland R. A., Henry J. L. Intrathecal administration of CGRP in the rat attenuates a facilitation of the tail flick reflex induced by either substance P or noxious cutaneous stimulation. Neurosci Lett. 1989 Jul 31;102(2-3):241–246. doi: 10.1016/0304-3940(89)90085-2. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Perkins M. N., Blake D. R., Kidd B. L. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994 Nov;113(3):940–944. doi: 10.1111/j.1476-5381.1994.tb17083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko C. W., Petricevic M. Sympathetic or reflex footpad swelling due to crystal-induced inflammation in the opposite foot. Inflammation. 1978 Mar;3(1):81–86. doi: 10.1007/BF00917323. [DOI] [PubMed] [Google Scholar]
- Dennis T., Fournier A., Cadieux A., Pomerleau F., Jolicoeur F. B., St Pierre S., Quirion R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J Pharmacol Exp Ther. 1990 Jul;254(1):123–128. [PubMed] [Google Scholar]
- Donnerer J., Stein C. Evidence for an increase in the release of CGRP from sensory nerves during inflammation. Ann N Y Acad Sci. 1992 Jun 30;657:505–506. doi: 10.1111/j.1749-6632.1992.tb22814.x. [DOI] [PubMed] [Google Scholar]
- Draisci G., Kajander K. C., Dubner R., Bennett G. J., Iadarola M. J. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res. 1991 Sep 27;560(1-2):186–192. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- Garry M. G., Hargreaves K. M. Enhanced release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carrageenan inflammation. Brain Res. 1992 Jun 5;582(1):139–142. doi: 10.1016/0006-8993(92)90328-7. [DOI] [PubMed] [Google Scholar]
- Hanesch U., Pfrommer U., Grubb B. D., Heppelmann B., Schaible H. G. The proportion of CGRP-immunoreactive and SP-mRNA containing dorsal root ganglion cells is increased by a unilateral inflammation of the ankle joint of the rat. Regul Pept. 1993 Jul 2;46(1-2):202–203. doi: 10.1016/0167-0115(93)90033-5. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Arvidsson U., Ceccatelli S., Cortés R., Cullheim S., Dagerlind A., Johnson H., Orazzo C., Piehl F., Pieribone V. Calcitonin gene-related peptide in the brain, spinal cord, and some peripheral systems. Ann N Y Acad Sci. 1992 Jun 30;657:119–134. doi: 10.1111/j.1749-6632.1992.tb22762.x. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A., Tohyama M. Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol. 1989;33(5-6):335–386. doi: 10.1016/0301-0082(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Neuropeptides: promise unfulfilled? Trends Neurosci. 1995 Feb;18(2):49–50. [PubMed] [Google Scholar]
- Kawamura M., Kuraishi Y., Minami M., Satoh M. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res. 1989 Sep 11;497(1):199–203. doi: 10.1016/0006-8993(89)90990-6. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y., Nanayama T., Ohno H., Minami M., Satoh M. Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett. 1988 Oct 17;92(3):325–329. doi: 10.1016/0304-3940(88)90611-8. [DOI] [PubMed] [Google Scholar]
- Le Greves P., Nyberg F., Terenius L., Hökfelt T. Calcitonin gene-related peptide is a potent inhibitor of substance P degradation. Eur J Pharmacol. 1985 Sep 24;115(2-3):309–311. doi: 10.1016/0014-2999(85)90706-x. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Opioid control of the function of primary afferent substance P fibres. Eur J Pharmacol. 1985 Aug 27;114(3):241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Basbaum A. I., Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985 May;5(5):1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg T., Meister B., Björkstrand E., Uvnäs-Moberg K. Oxytocin modulates the effects of galanin in carrageenan-induced hyperalgesia in rats. Brain Res. 1993 Apr 16;608(2):181–185. doi: 10.1016/0006-8993(93)91456-3. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Chiba T., Giuliani S. Human alpha-calcitonin gene-related peptide-(8-37) as an antagonist of exogenous and endogenous calcitonin gene-related peptide. Eur J Pharmacol. 1991 Jan 3;192(1):85–88. doi: 10.1016/0014-2999(91)90072-x. [DOI] [PubMed] [Google Scholar]
- Mao J., Coghill R. C., Kellstein D. E., Frenk H., Mayer D. J. Calcitonin gene-related peptide enhances substance P-induced behaviors via metabolic inhibition: in vivo evidence for a new mechanism of neuromodulation. Brain Res. 1992 Mar 6;574(1-2):157–163. doi: 10.1016/0006-8993(92)90812-n. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Terenghi G., Walsh D. A., Chen S. T., Cruwys S. C., Garrett N., Kidd B. L., Polak J. M., Blake D. R. Monoarthritis in the rat knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neuroscience. 1993 Dec;57(4):1091–1096. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Walsh D. A., Kidd B. L., Cruwys S. C., Polak J. M., Blake D. R. Localization of the enzyme neutral endopeptidase to the human synovium. J Rheumatol. 1992 Dec;19(12):1838–1844. [PubMed] [Google Scholar]
- Mayer E. A., Raybould H., Koelbel C. Neuropeptides, inflammation, and motility. Dig Dis Sci. 1988 Mar;33(3 Suppl):71S–77S. doi: 10.1007/BF01538134. [DOI] [PubMed] [Google Scholar]
- McNeill D. L., Chung K., Carlton S. M., Coggeshall R. E. Calcitonin gene-related peptide immunostained axons provide evidence for fine primary afferent fibers in the dorsal and dorsolateral funiculi of the rat spinal cord. J Comp Neurol. 1988 Jun 8;272(2):303–308. doi: 10.1002/cne.902720212. [DOI] [PubMed] [Google Scholar]
- Nanayama T., Kuraishi Y., Ohno H., Satoh M. Capsaicin-induced release of calcitonin gene-related peptide from dorsal horn slices is enhanced in adjuvant arthritic rats. Neurosci Res. 1989 Aug;6(6):569–572. doi: 10.1016/0168-0102(89)90045-x. [DOI] [PubMed] [Google Scholar]
- Oku R., Satoh M., Fujii N., Otaka A., Yajima H., Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987 Feb 17;403(2):350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- Pohl M., Mauborgne A., Bourgoin S., Benoliel J. J., Hamon M., Cesselin F. Neonatal capsaicin treatment abolishes the modulations by opioids of substance P release from rat spinal cord slices. Neurosci Lett. 1989 Jan 2;96(1):102–107. doi: 10.1016/0304-3940(89)90250-4. [DOI] [PubMed] [Google Scholar]
- Poyner D. R. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacol Ther. 1992;56(1):23–51. doi: 10.1016/0163-7258(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Ryu P. D., Gerber G., Murase K., Randic M. Actions of calcitonin gene-related peptide on rat spinal dorsal horn neurons. Brain Res. 1988 Feb 16;441(1-2):357–361. doi: 10.1016/0006-8993(88)91414-x. [DOI] [PubMed] [Google Scholar]
- Satoh M., Kuraishi Y., Kawamura M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain. 1992 May;49(2):273–278. doi: 10.1016/0304-3959(92)90151-Z. [DOI] [PubMed] [Google Scholar]
- Takahashi O., Shiosaka S., Traub R. J., Ruda M. A. Ultrastructural demonstration of synaptic connections between calcitonin gene-related peptide immunoreactive axons and dynorphin A(1-8) immunoreactive dorsal horn neurons in a rat model of peripheral inflammation and hyperalgesia. Peptides. 1990 Nov-Dec;11(6):1233–1237. doi: 10.1016/0196-9781(90)90157-z. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E., Hemsén A., Brodin E., Lundberg J. M. Sample handling techniques when analyzing regulatory peptides. Life Sci. 1987 Aug 17;41(7):845–848. doi: 10.1016/0024-3205(87)90177-9. [DOI] [PubMed] [Google Scholar]
- Tschopp F. A., Henke H., Petermann J. B., Tobler P. H., Janzer R., Hökfelt T., Lundberg J. M., Cuello C., Fischer J. A. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci U S A. 1985 Jan;82(1):248–252. doi: 10.1073/pnas.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969 Mar;166(1):96–103. [PubMed] [Google Scholar]
- WINTER C. A., RISLEY E. A., NUSS G. W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962 Dec;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Hökfelt T., Lundberg J. M., Forssmann W. G., Reinecke M., Tschopp F. A., Fischer J. A. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett. 1984 Nov 23;52(1-2):199–204. doi: 10.1016/0304-3940(84)90374-4. [DOI] [PubMed] [Google Scholar]
- Woolf C., Wiesenfeld-Hallin Z. Substance P and calcitonin gene-related peptide synergistically modulate the gain of the nociceptive flexor withdrawal reflex in the rat. Neurosci Lett. 1986 May 15;66(2):226–230. doi: 10.1016/0304-3940(86)90195-3. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Hansson P., Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res. 1994 Aug 8;653(1-2):223–230. doi: 10.1016/0006-8993(94)90393-x. [DOI] [PubMed] [Google Scholar]



