Abstract
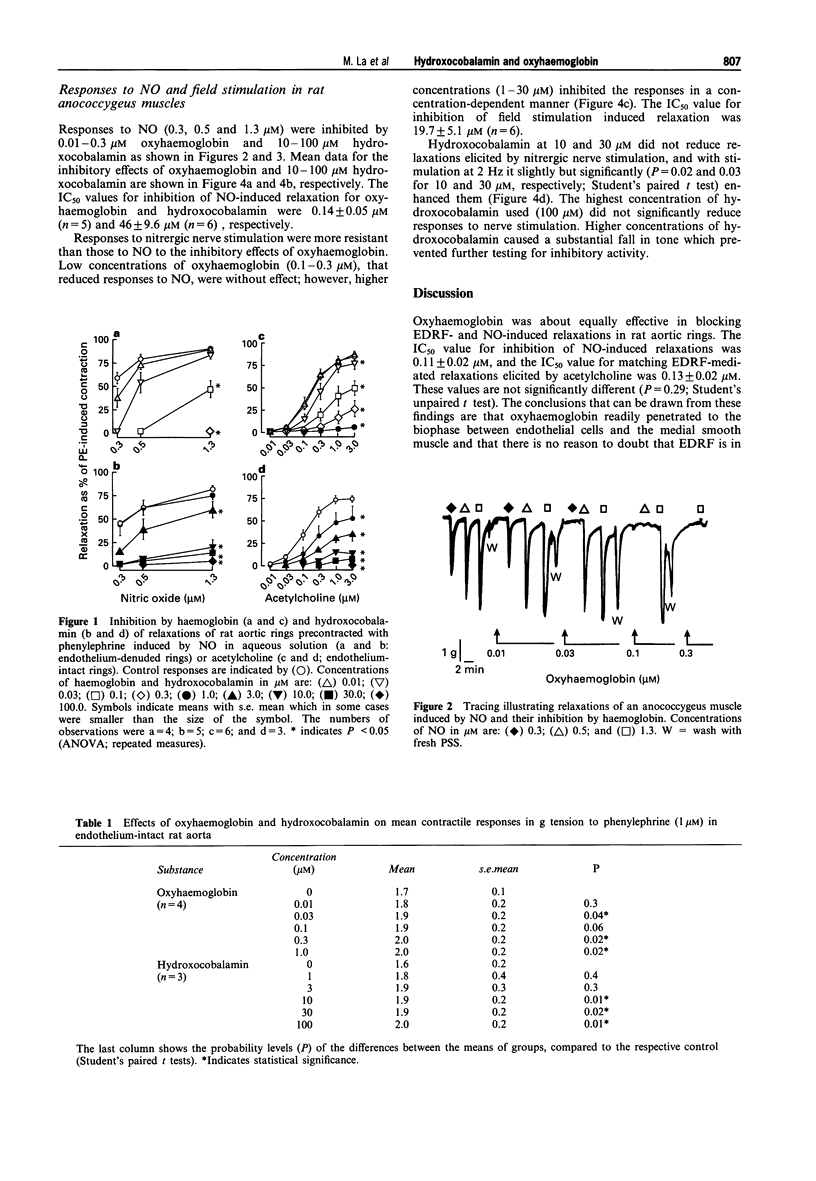
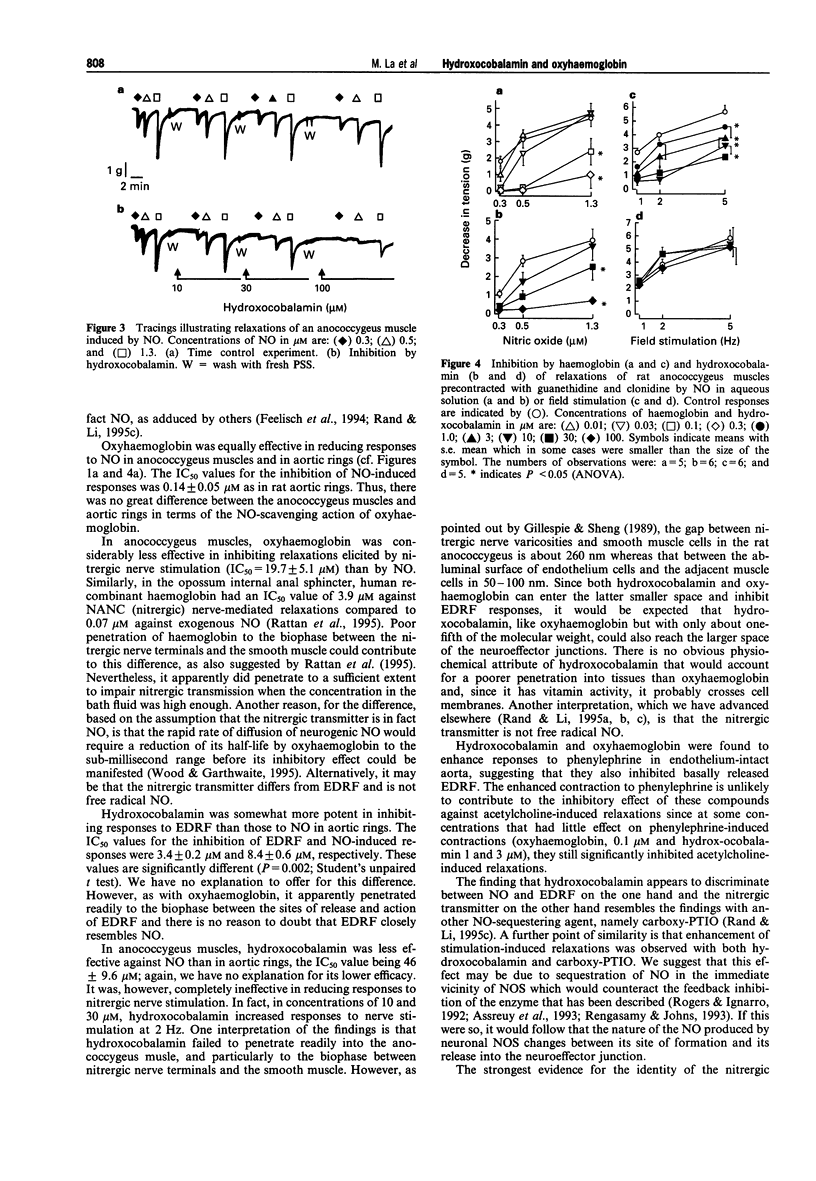
1. The effects of ranges of concentrations of oxyhaemoglobin (0.01-30 microM) and hydroxocobalamin (1-100 microM) were compared for their abilities to reduce relaxant responses to EDRF released by acetylcholine in endothelium-intact rat aortic rings, the nitergic transmitter in rat anococcygeus muscles, and NO in aqueous solution in both tissues (aortic rings were denuded of endothelium). 2. The concentrations of oxyhaemoglobin producing 50% reduction of responses to EDRF and NO in rat aorta correspond closely, the IC50 values being 0.13 +/- 0.02 microM and 0.11 +/- 0.02 microM respectively. 3. Oxyhaemoglobin was equally effective in inhibiting responses to NO in anococcygeus muscles and in aortic rings with an IC50 of 0.14 +/- 0.05 microM. However, responses to the nitrergic transmitter were considerably less sensitive to inhibition by oxyhaemoglobin, the IC50 being 19.7 +/- 5.1 microM. 4. The IC50 values for hydroxocobalamin in inhibiting responses to EDRF and NO in aorta were 3.4 +/- 0.2 microM and 8.4 +/- 0.63 microM, respectively, but it was less effective against responses to NO in anococcygeus muscles the IC50 being 46 +/- 9.6 microM. However, even in the highest concentration used (100 microM), it did not reduce responses to the nitrergic transmitter. 5. The findings are compatible with the views that EDRF is NO, but suggest that the nitergic transmitter in the rat anococcygeus muscle does not behave like free NO.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assreuy J., Cunha F. Q., Liew F. Y., Moncada S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993 Mar;108(3):833–837. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. N., Harrison D. G., Myers P. R., Minor R. L. EDRF: nitrosylated compound or authentic nitric oxide. Basic Res Cardiol. 1991;86 (Suppl 2):17–26. doi: 10.1007/978-3-642-72461-9_3. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., De Man J. G., De Winter B. Y., Herman A. G., Pelckmans P. A. Pharmacological similarity between nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Eur J Pharmacol. 1994 Oct 13;264(1):85–89. doi: 10.1016/0014-2999(94)90640-8. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Ruytjens I. F., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br J Pharmacol. 1991 May;103(1):1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- De Man J. G., Boeckxstaens G. E., De Winter B. Y., Moreels T. G., Misset M. E., Herman A. G., Pelckmans P. A. Comparison of the pharmacological profile of S-nitrosothiols, nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Br J Pharmacol. 1995 Mar;114(6):1179–1184. doi: 10.1111/j.1476-5381.1995.tb13331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957 May 23;136(3):507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. A comparison of haemoglobin and erythrocytes as inhibitors of smooth muscle relaxation by the NANC transmitter in the BRP and rat anococcygeus and by EDRF in the rabbit aortic strip. Br J Pharmacol. 1989 Oct;98(2):445–450. doi: 10.1111/j.1476-5381.1989.tb12616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. Influence of haemoglobin and erythrocytes on the effects of EDRF, a smooth muscle inhibitory factor, and nitric oxide on vascular and non-vascular smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1151–1156. doi: 10.1111/j.1476-5381.1988.tb11750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen H. H., Wiklund N. P., Gustafsson L. E. Nitric oxide-like activity in guinea pig colon as determined by effector responses, bioassay and chemiluminescence analysis. Acta Physiol Scand. 1994 Nov;152(3):315–322. doi: 10.1111/j.1748-1716.1994.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson K. M., Reid J. J., Rand M. J. Hydroxocobalamin and haemoglobin differentiate between exogenous and neuronal nitric oxide in the rat gastric fundus. Eur J Pharmacol. 1995 Mar 6;275(2):145–152. doi: 10.1016/0014-2999(94)00762-v. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Effects of hydroxocobalamin and haemoglobin on no-mediated relaxations in the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1993 Oct;20(10):633–640. doi: 10.1111/j.1440-1681.1993.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanayagam M. A., Li C. G., Rand M. J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br J Pharmacol. 1993 Jan;108(1):3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Differential effects of hydroxocobalamin on relaxations induced by nitrosothiols in rat aorta and anococcygeus muscle. Eur J Pharmacol. 1993 Sep 14;241(2-3):249–254. doi: 10.1016/0014-2999(93)90210-9. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br J Pharmacol. 1995 Sep;116(2):1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Rattan S., Rosenthal G. J., Chakder S. Human recombinant hemoglobin (rHb1.1) inhibits nonadrenergic noncholinergic (NANC) nerve-mediated relaxation of internal anal sphincter. J Pharmacol Exp Ther. 1995 Mar;272(3):1211–1216. [PubMed] [Google Scholar]
- Rengasamy A., Johns R. A. Regulation of nitric oxide synthase by nitric oxide. Mol Pharmacol. 1993 Jul;44(1):124–128. [PubMed] [Google Scholar]
- Rogers N. E., Ignarro L. J. Constitutive nitric oxide synthase from cerebellum is reversibly inhibited by nitric oxide formed from L-arginine. Biochem Biophys Res Commun. 1992 Nov 30;189(1):242–249. doi: 10.1016/0006-291x(92)91550-a. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Mordvintcev P. I., Malenkova I. V., Vanin A. F. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur J Pharmacol. 1992 Feb 18;211(3):313–317. doi: 10.1016/0014-2999(92)90386-i. [DOI] [PubMed] [Google Scholar]
- Wood J., Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994 Nov;33(11):1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


