Abstract
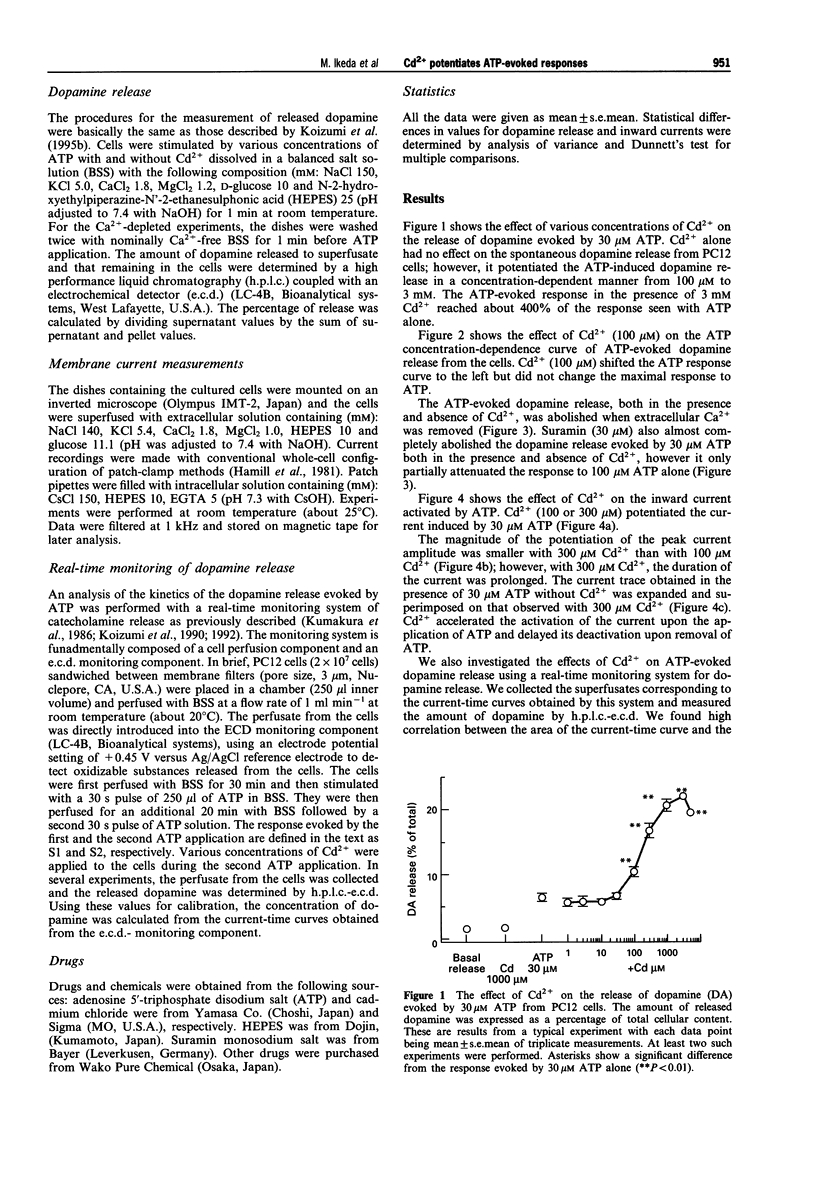
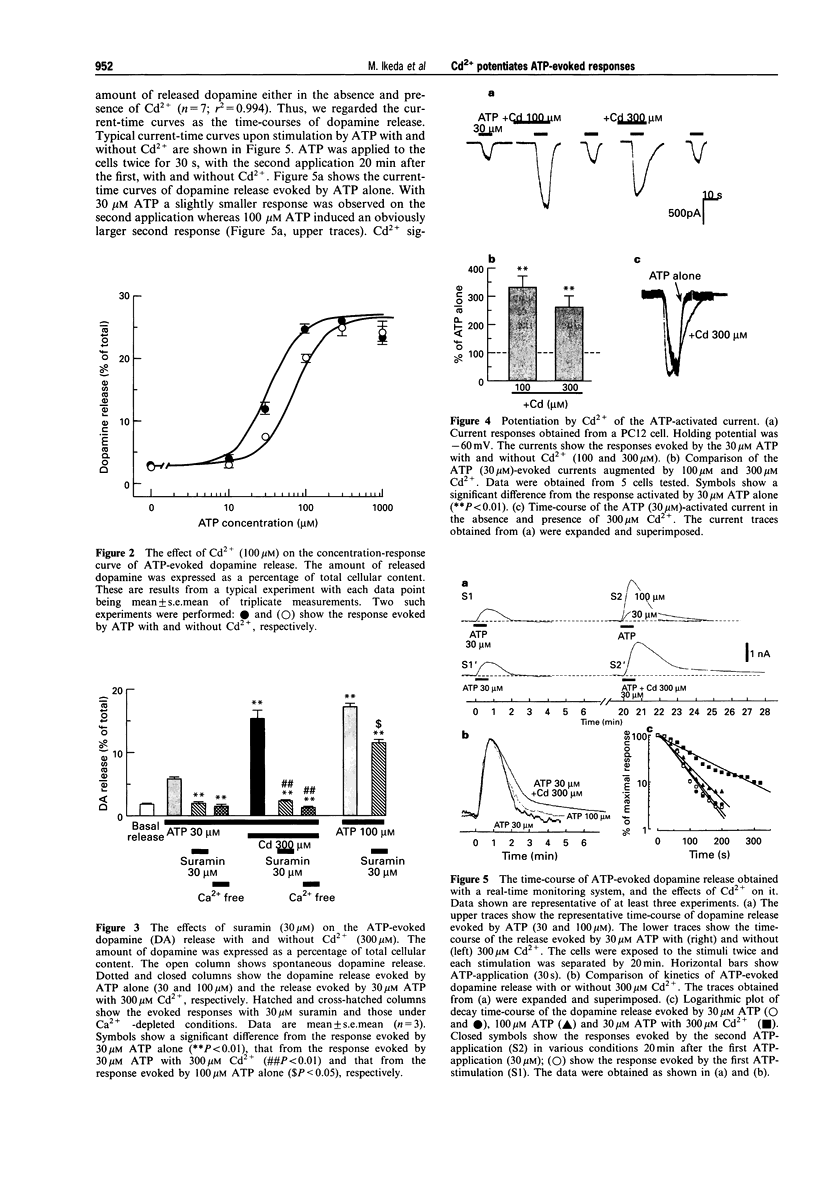
1. The effects of cadmium ion (Cd2+) on release of dopamine and on an inward current evoked by extracellular ATP were investigated in rat phaeochromocytoma PC12 cells. 2. Cd2+ (100 microM-3 mM) potentiated the dopamine release evoked by 30 microM ATP from the cells. Cd2+ (100 microM) shifted the concentration-response curve of ATP-evoked dopamine release to the left without affecting the maximal response. 3. Suramin (30 microM) completely abolished the dopamine release evoked by 30 microM ATP but only partially inhibited the release evoked by 100 microM ATP consistent with its role as a competitive antagonist. The response evoked by 30 microM ATP in the presence of Cd2+ (300 microM) was comparable to that observed with 100 microM ATP alone; however, only the former was almost completely inhibited by suramin. 4. Cd2+ (100 microM) potentiated an inward current activated by 30 microM ATP alone. A higher concentration of Cd2+ (300 microM) had a smaller effect on amplitude potentiation but significantly prolonged the duration of the current. 5. The time-course of the ATP-evoked dopamine release was investigated using a real-time monitoring system for dopamine release. Although Cd2+ (300 microM) had little effect on the time-course of activation the ATP-evoked dopamine release, it produced a long-lasting dopamine release which slowly returned to the baseline. 6. Taken together, these observations suggest that Cd2+ enhances ATP-evoked dopamine release by affecting P2-purinoceptor/channels. The enhancement may be attributed to a Cd(2+)-dependent increase in sensitivity to ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Friel D. D. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Chaube S., Nishimura H., Swinyard C. A. Zinc and cadmium in normal human embryos and fetuses: analyses by atomic absorption spectrophotometry. Arch Environ Health. 1973 May;26(5):237–240. doi: 10.1080/00039896.1973.10666265. [DOI] [PubMed] [Google Scholar]
- Cloues R., Jones S., Brown D. A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflugers Arch. 1993 Jul;424(2):152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honoré E., Martin C., Mironneau C., Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989 Aug;257(2 Pt 1):C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- Inoue K., Kenimer J. G. Muscarinic stimulation of calcium influx and norepinephrine release in PC12 cells. J Biol Chem. 1988 Jun 15;263(17):8157–8161. [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett. 1989 Dec 4;106(3):294–299. doi: 10.1016/0304-3940(89)90179-1. [DOI] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Watano T., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked glutamate release in cultured hippocampal neurons. Neurosci Lett. 1992 Jan 6;134(2):215–218. doi: 10.1016/0304-3940(92)90520-h. [DOI] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Watano T., Ohara-Imaizumi M., Fujimori K., Takanaka A. Dopamine receptor agonists and antagonists enhance ATP-activated currents. Eur J Pharmacol. 1992 May 14;215(2-3):321–324. doi: 10.1016/0014-2999(92)90049-a. [DOI] [PubMed] [Google Scholar]
- Inoue K., Watano T., Koizumi S., Nakazawa K., Burnstock G. Dual modulation by adenosine of ATP-activated channels through GTP-binding proteins in rat pheochromocytoma PC12 cells. Eur J Pharmacol. 1994 Jul 15;268(2):223–229. doi: 10.1016/0922-4106(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Ohara-Imaizumi M., Ueki S., Kumakura K. Stimulatory action of gamma-aminobutyric acid on catecholamine secretion from bovine adrenal chromaffin cells measured by a real-time monitoring system. J Neurochem. 1988 Jun;50(6):1765–1768. doi: 10.1111/j.1471-4159.1988.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Kiss T., Osipenko O. N. Toxic effects of heavy metals on ionic channels. Pharmacol Rev. 1994 Sep;46(3):245–267. [PubMed] [Google Scholar]
- Koizumi S., Ikeda M., Inoue K., Nakazawa K., Inoue K. Enhancement by zinc of ATP-evoked dopamine release from rat pheochromocytoma PC12 cells. Brain Res. 1995 Feb 27;673(1):75–82. doi: 10.1016/0006-8993(94)01404-6. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Ikeda M., Nakazawa K., Inoue K., Nagamatsu K., Hasegawa A., Inoue K. Accentuation by pertussis toxin of the 5-hydroxytryptamine-induced potentiation of ATP-evoked responses in rat pheochromocytoma cells. Neurosci Lett. 1995 Jan 2;183(1-2):104–107. doi: 10.1016/0304-3940(94)11125-3. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Kataoka Y., Niwa M., Kumakura K. Endothelin-3 stimulates the release of catecholamine from cortical and striatal slices of the rat. Neurosci Lett. 1992 Jan 6;134(2):219–222. doi: 10.1016/0304-3940(92)90521-8. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Kataoka Y., Shigematsu K., Niwa M., Ueki S. Evaluation of the neuroprotective action of WEB 1881 FU on hypoglycemia/hypoxia-induced neuronal damage using rat striatal slices. Jpn J Pharmacol. 1990 Jun;53(2):175–183. doi: 10.1254/jjp.53.175. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Watano T., Nakazawa K., Inoue K. Potentiation by adenosine of ATP-evoked dopamine release via a pertussis toxin-sensitive mechanism in rat phaeochromocytoma PC12 cells. Br J Pharmacol. 1994 Jul;112(3):992–997. doi: 10.1111/j.1476-5381.1994.tb13179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Li Z., Weight F. F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8264–8267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Murphy V. A., Embrey E. C., Rosenberg J. M., Smith Q. R., Rapoport S. I. Calcium deficiency enhances cadmium accumulation in the central nervous system. Brain Res. 1991 Aug 23;557(1-2):280–284. doi: 10.1016/0006-8993(91)90144-k. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Hess P. Block by calcium of ATP-activated channels in pheochromocytoma cells. J Gen Physiol. 1993 Mar;101(3):377–392. doi: 10.1085/jgp.101.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K., Koizumi S., Inoue K. Facilitation by 5-hydroxytryptamine of ATP-activated current in rat pheochromocytoma cells. Pflugers Arch. 1994 Jul;427(5-6):492–499. doi: 10.1007/BF00374266. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K. Roles of Ca2+ influx through ATP-activated channels in catecholamine release from pheochromocytoma PC12 cells. J Neurophysiol. 1992 Dec;68(6):2026–2032. doi: 10.1152/jn.1992.68.6.2026. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Watano T., Inoue K. Mechanisms underlying facilitation by dopamine of ATP-activated currents in rat pheochromocytoma cells. Pflugers Arch. 1993 Feb;422(5):458–464. doi: 10.1007/BF00375072. [DOI] [PubMed] [Google Scholar]
- Nelson M. T. Interactions of divalent cations with single calcium channels from rat brain synaptosomes. J Gen Physiol. 1986 Feb;87(2):201–222. doi: 10.1085/jgp.87.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Dwyer S. D., Smith L. Cadmium evokes inositol polyphosphate formation and calcium mobilization. Evidence for a cell surface receptor that cadmium stimulates and zinc antagonizes. J Biol Chem. 1989 May 5;264(13):7115–7118. [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Coogan T. P., Barter R. A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22(3-4):175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]



