Abstract
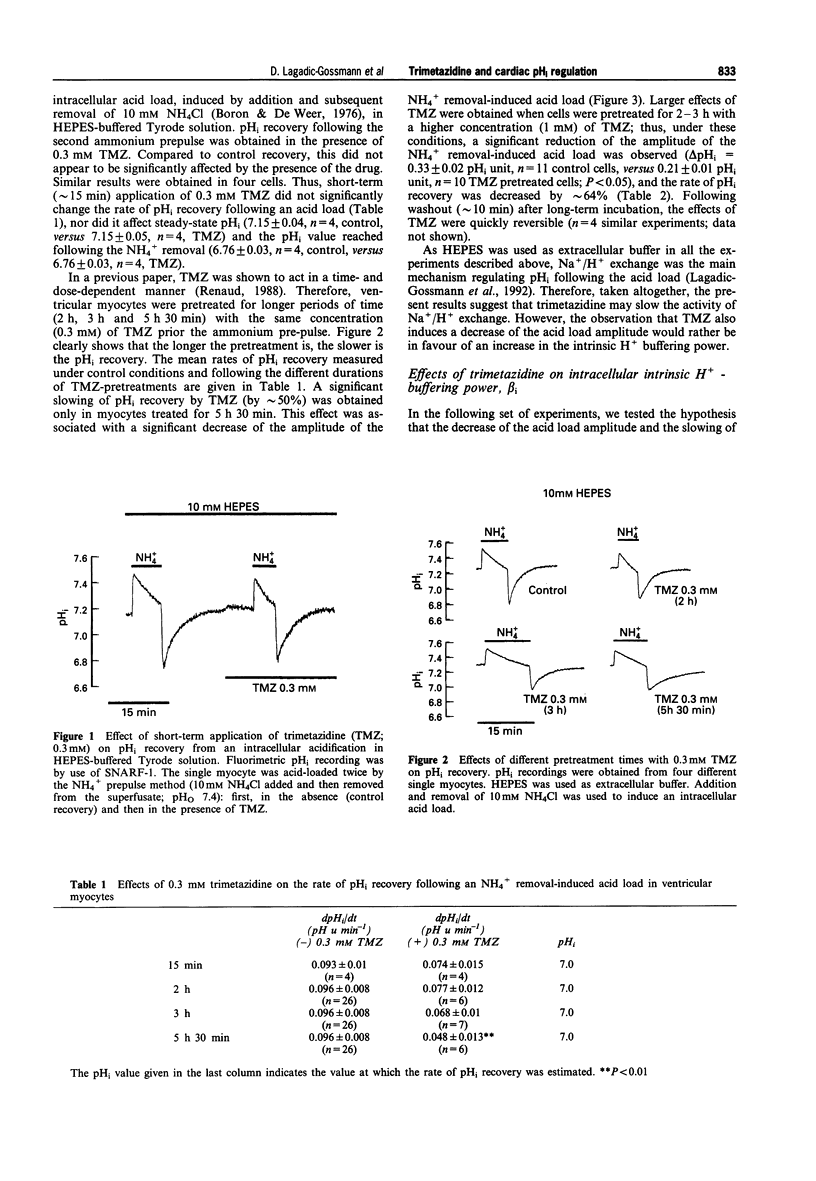
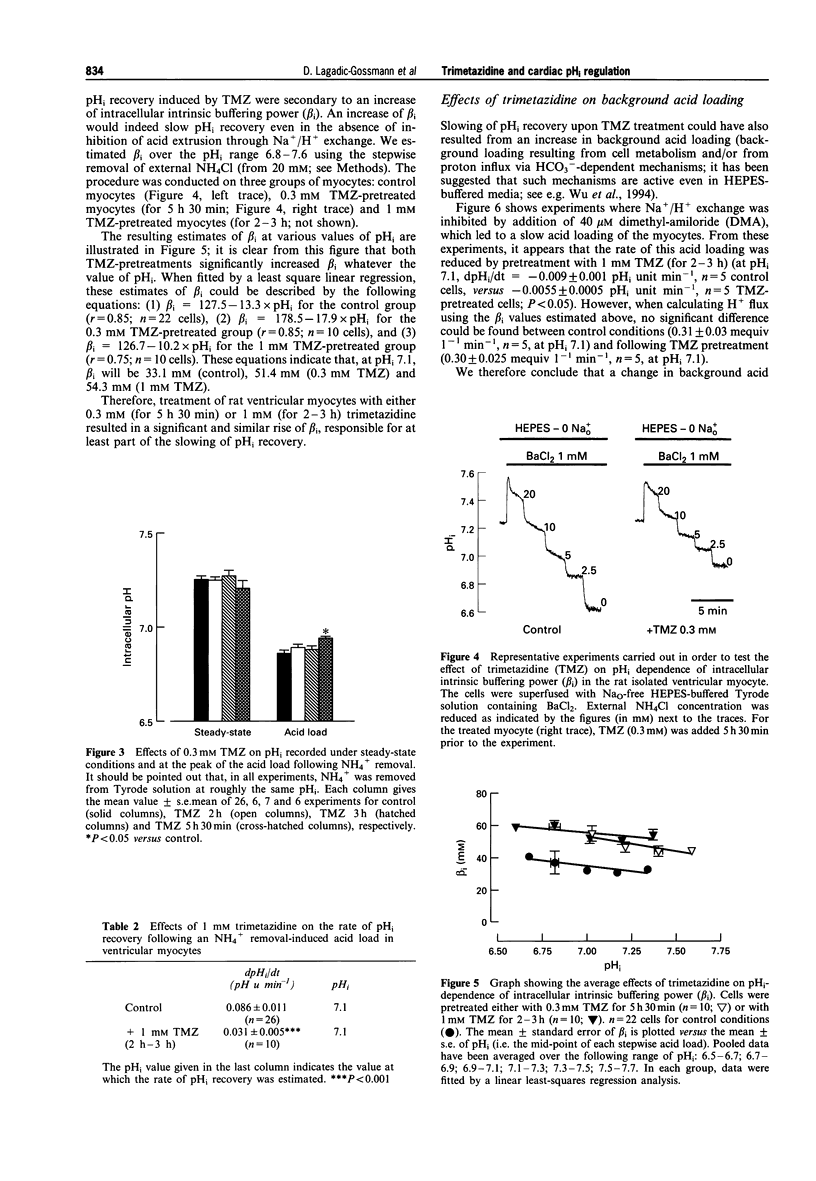
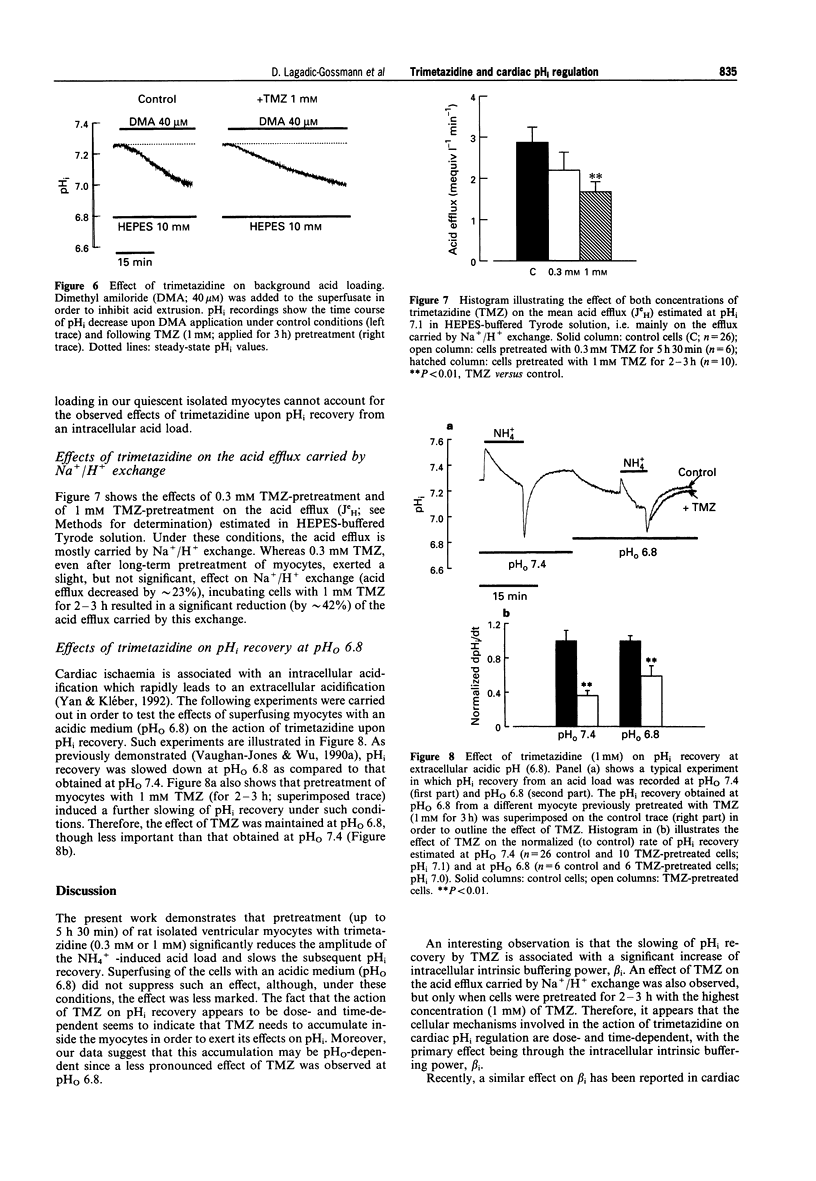
1. We have examined the effects of trimetazidine (TMZ) on intracellular pH (pHi) regulation in rat isolated ventricular myocytes. pHi was recorded ratiometrically by use of the pH-sensitive fluoroprobe, carboxy-SNARF-1 (carboxy-seminaphtorhodafluor). 2. Following an intracellular acid load (induced by 10 mM NH4Cl removal), pHi recovery in HEPES-buffered Tyrode solution was significantly slowed down upon application of 0.3 mM TMZ only when myocytes were pretreated for 5 h 30 min (slowing by approximately 50%; P < 0.01). This effect of TMZ on pHi recovery was shown to be not only time- but also dose-dependent with a large, quickly reversible, effect obtained with 1 mM TMZ applied for 2-3 h (slowing by approximately 64%; P < 0.001). This slowing of pHi recovery was also associated with a decrease of the NH4+ removal-induced acidification. 3. Relationship between intracellular intrinsic buffering power (beta i) and pHi was assessed in absence or presence of TMZ (0.3 mM or 1 mM). As expected, beta i increased roughly linearly with a decrease in pHi in all cases. However, both concentrations of TMZ significantly increased beta i (by approximately 55 and 65% at pHi 7.1, respectively). 4. When Na+/H+ exchange was inhibited by dimethyl amiloride (DMA; 40 microM), trimetazidine (1 mM) did not change the H+ flux estimated at pHi 7.1 (0.31 +/- 0.03 mequiv l-1 min-1, n = 5, control, versus 0.30 +/- 0.025 mequiv l-1 min-1, n = 5, TMZ), ruling out any effect of TMZ on background acid loading. 5. Acid efflux carried by Na+/H+ exchange was significantly decreased only when myocytes were pretreated with 1 mM TMZ, for 2-3 h (JeH = 2.86 +/- 0.38 mequiv l-1 min-1, n = 26, control, versus 1.66 +/- 0.26 mequiv l-1 min-1, n = 10, TMZ, estimated at pHi 7.1; P < 0.05). 6. In conclusion, the present work demonstrates that, following an intracellular acid load in HEPES-buffered medium, trimetazidine slows down pHi recovery in rat isolated ventricular myocytes, primarily through an increase of beta i. An effect on Na+/H+ exchange is also detected but only after long-term incubation of the myocytes with TMZ.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aussedat J., Ray A., Kay L., Verdys M., Harpey C., Rossi A. Improvement of long-term preservation of isolated arrested rat heart: beneficial effect of the antiischemic agent trimetazidine. J Cardiovasc Pharmacol. 1993 Jan;21(1):128–135. doi: 10.1097/00005344-199301000-00019. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher F. R., Hearse D. J., Opie L. H. Effects of trimetazidine on ischemic contracture in isolated perfused rat hearts. J Cardiovasc Pharmacol. 1994 Jul;24(1):45–49. doi: 10.1097/00005344-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Counillon L., Pouysségur J. Structure-function studies and molecular regulation of the growth factor activatable sodium-hydrogen exchanger (NHE-1). Cardiovasc Res. 1995 Feb;29(2):147–154. [PubMed] [Google Scholar]
- Dennis S. C., Gevers W., Opie L. H. Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol. 1991 Sep;23(9):1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- Devynck M. A., Le Quan Sang K. H., Joulin Y., Mazeaud M. Acute membrane effects of trimetazidine in human platelets. Eur J Pharmacol. 1993 Apr 15;245(2):105–110. doi: 10.1016/0922-4106(93)90117-r. [DOI] [PubMed] [Google Scholar]
- Dudeja P. K., Foster E. S., Brasitus T. A. Modulation of rat distal colonic brush-border membrane Na+-H+ exchange by dexamethasone: role of lipid fluidity. Biochim Biophys Acta. 1987 Dec 11;905(2):485–493. doi: 10.1016/0005-2736(87)90478-0. [DOI] [PubMed] [Google Scholar]
- Fabiani J. N., Ponzio O., Emerit I., Massonet-Castel S., Paris M., Chevalier P., Jebara V., Carpentier A. Cardioprotective effect of trimetazidine during coronary artery graft surgery. J Cardiovasc Surg (Torino) 1992 Jul-Aug;33(4):486–491. [PubMed] [Google Scholar]
- Fliegel L., Fröhlich O. The Na+/H+ exchanger: an update on structure, regulation and cardiac physiology. Biochem J. 1993 Dec 1;296(Pt 2):273–285. doi: 10.1042/bj2960273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Goetz J. D., Rothstein A. 22Na+ fluxes in thymic lymphocytes. II. Amiloride-sensitive Na+/H+ exchange pathway; reversibility of transport and asymmetry of the modifier site. J Gen Physiol. 1984 Oct;84(4):585–600. doi: 10.1085/jgp.84.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatome I., Ishiko R., Tanaka Y., Kosaka H., Hasegawa J., Yoshida A., Kotake H., Mashiba H., Arita M. Trimetazidine inhibits Na+,K(+)-ATPase activity, and overdrive hyperpolarization in guinea-pig ventricular muscles. Eur J Pharmacol. 1991 Apr 3;195(3):381–388. doi: 10.1016/0014-2999(91)90479-a. [DOI] [PubMed] [Google Scholar]
- Jourdon P., Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993 Oct;470:411–429. doi: 10.1113/jphysiol.1993.sp019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn M., Moffat M. P. Role of Na+/H+ exchange in cardiac physiology and pathophysiology: mediation of myocardial reperfusion injury by the pH paradox. Cardiovasc Res. 1993 Jun;27(6):915–924. doi: 10.1093/cvr/27.6.915. [DOI] [PubMed] [Google Scholar]
- Khandoudi N., Bernard M., Cozzone P., Feuvray D. Intracellular pH and role of Na+/H+ exchange during ischaemia and reperfusion of normal and diabetic rat hearts. Cardiovasc Res. 1990 Nov;24(11):873–878. doi: 10.1093/cvr/24.11.873. [DOI] [PubMed] [Google Scholar]
- Kober G., Buck T., Sievert H., Vallbracht C. Myocardial protection during percutaneous transluminal coronary angioplasty: effects of trimetazidine. Eur Heart J. 1992 Aug;13(8):1109–1115. doi: 10.1093/oxfordjournals.eurheartj.a060322. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Buckler K. J., Vaughan-Jones R. D. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanchy N., Martin J., Rossi A. Anti-ischemic effects of trimetazidine: 31P-NMR spectroscopy in the isolated rat heart. Arch Int Pharmacodyn Ther. 1987 Mar;286(1):97–110. [PubMed] [Google Scholar]
- Murphy E., Perlman M., London R. E., Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991 May;68(5):1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Grotyohann L. W. Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ Res. 1984 Dec;55(6):816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- Noël J., Pouysségur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. Am J Physiol. 1995 Feb;268(2 Pt 1):C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Czubryt M. P. The contribution of ionic imbalance to ischemia/reperfusion-induced injury. J Mol Cell Cardiol. 1995 Jan;27(1):53–63. doi: 10.1016/s0022-2828(08)80007-7. [DOI] [PubMed] [Google Scholar]
- Renaud J. F. Internal pH, Na+, and Ca2+ regulation by trimetazidine during cardiac cell acidosis. Cardiovasc Drugs Ther. 1988 Mar;1(6):677–686. doi: 10.1007/BF02125756. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Tani M. Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol. 1990;52:543–559. doi: 10.1146/annurev.ph.52.030190.002551. [DOI] [PubMed] [Google Scholar]
- Tani M., Shinmura K., Ebihara Y., Asakura Y. Inhibition of glycolysis or increased perfusate H+ buffering capacity, but not their combination, attenuates myocardial stunning. Cardiovasc Res. 1993 Sep;27(9):1645–1650. doi: 10.1093/cvr/27.9.1645. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Acidification and intracellular sodium ion activity during stimulated myocardial ischemia. Am J Physiol. 1990 Jul;259(1 Pt 1):C169–C179. doi: 10.1152/ajpcell.1990.259.1.C169. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. L., Tsai M. L., Tseng Y. Z. DIDS-sensitive pHi regulation in single rat cardiac myocytes in nominally HCO3-free conditions. Circ Res. 1994 Jul;75(1):123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- Wu M. L., Vaughan-Jones R. D. Effect of metabolic inhibitors and second messengers upon Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1994 Jul 15;478(Pt 2):301–313. doi: 10.1113/jphysiol.1994.sp020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G. X., Kléber A. G. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ Res. 1992 Aug;71(2):460–470. doi: 10.1161/01.res.71.2.460. [DOI] [PubMed] [Google Scholar]


