Abstract
1. The involvement of beta 1-, beta 2- and beta 3-adrenoceptors in the control of lipolysis and nutritive blood flow was investigated in abdominal subcutaneous adipose tissue of healthy young adults by use of an in situ microdialysis technique. 2. Dialysis probes were infused either with isoprenaline (non-selective beta-adrenoceptor agonist), CGP 12,177 (selective beta 3-adrenoceptor agonist having beta 1-/beta 2-antagonist properties), dobutamine (selective beta 1-adrenoceptor agonist) or terbutaline (selective beta 2-adrenoceptor agonist). The recovery of each probe used for perfusion was calculated by an in vivo calibration method. The local blood flow was estimated through the measurement of the escape of ethanol infused simultaneously with the drugs included in the probe. 3. Isoprenaline infusion at 0.01 microM had a weak effect while higher concentrations of isoprenaline (0.1 and 1 microM) caused a rapid, sustained and concentration-dependent increase of glycerol outflow; the maximum increase was 306 +/- 34% with 1 microM. Isoprenaline also increased the nutritive blood flow in adipose tissue; a significant effect appeared at 0.1 microM isoprenaline and was greater at 1 microM. 4. CGP 12,177 (10 and 100 microM) increased the glycerol concentration in the dialysate (128 +/- 8 and 149 +/- 12%, respectively) and nutritive blood flow. Terbutaline and dobutamine (100 microM) both provoked rapid and similar increases in glycerol outflow (252 +/- 18 and 249 +/- 18%, respectively). Both, terbutaline and dobutamine increased nutritive blood flow. 5. It is concluded that beta 1- and beta 2-adrenoceptor subtypes are both mainly involved in the mobilization of lipids and in the control of nutritive blood flow. beta 3-Adrenoceptors play a weaker role in the control of lipolysis and nutritive blood flow in human subcutaneous abdominal adipose tissue.
Full text
PDF

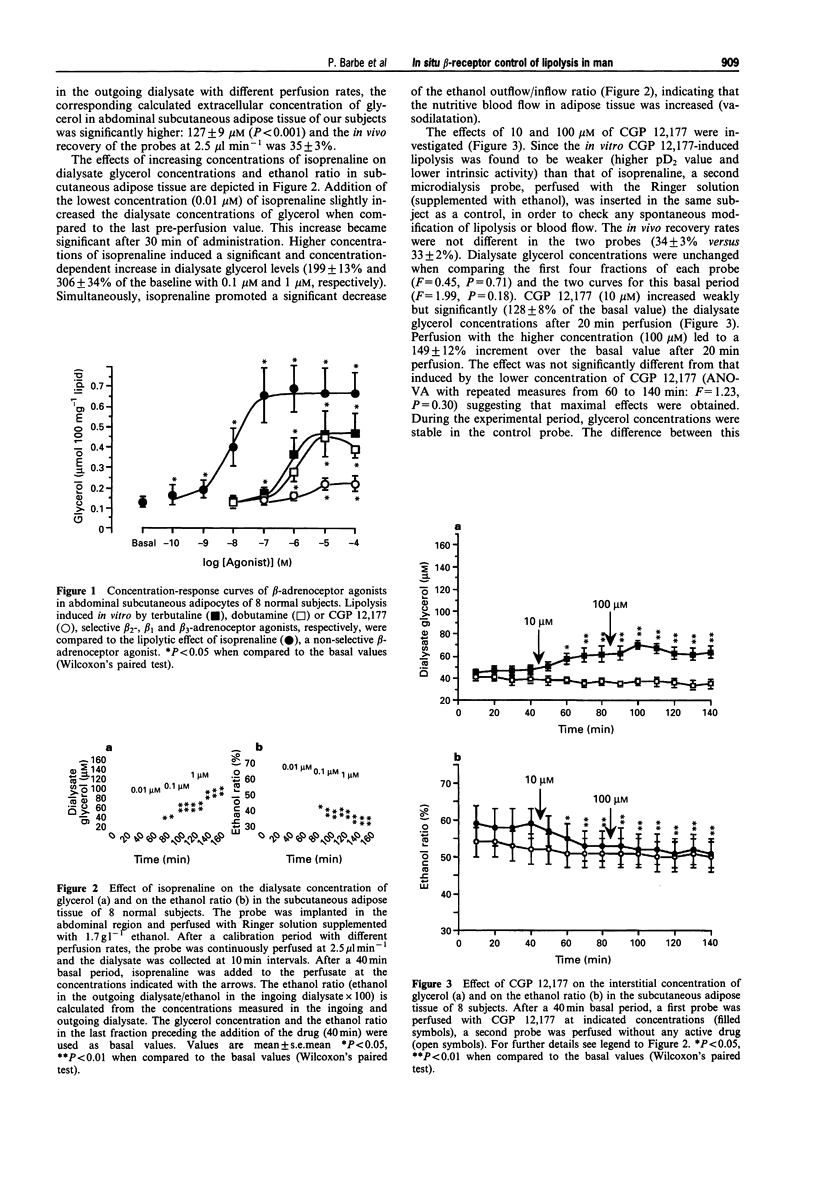
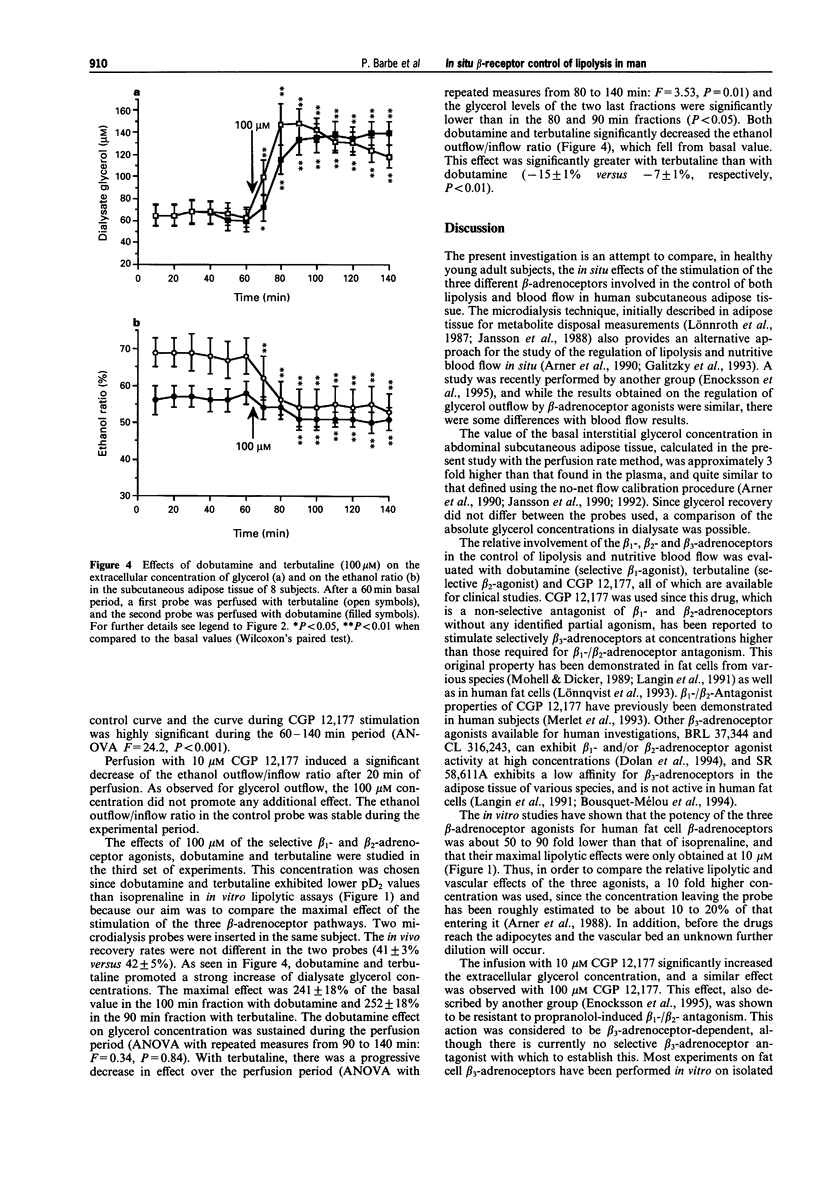



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992 Jan;55(1 Suppl):228S–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- Arner P., Bolinder J., Eliasson A., Lundin A., Ungerstedt U. Microdialysis of adipose tissue and blood for in vivo lipolysis studies. Am J Physiol. 1988 Nov;255(5 Pt 1):E737–E742. doi: 10.1152/ajpendo.1988.255.5.E737. [DOI] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Berlan M., Galitzky J., Bousquet-Melou A., Lafontan M., Montastruc J. L. Beta-3 adrenoceptor-mediated increase in cutaneous blood flow in the dog. J Pharmacol Exp Ther. 1994 Mar;268(3):1444–1451. [PubMed] [Google Scholar]
- Bousquet-Mélou A., Galitzky J., Carpéné C., Lafontan M., Berlan M. beta-Adrenergic control of lipolysis in primate white fat cells: a comparative study with nonprimate mammals. Am J Physiol. 1994 Jul;267(1 Pt 2):R115–R123. doi: 10.1152/ajpregu.1994.267.1.R115. [DOI] [PubMed] [Google Scholar]
- Bousquet-Mélou A., Galitzky J., Moreno C. M., Berlan M., Lafontan M. Desensitization of beta-adrenergic responses in adipocytes involves receptor subtypes and cAMP phosphodiesterase. Eur J Pharmacol. 1995 Apr 28;289(2):235–247. doi: 10.1016/0922-4106(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. C., Kaslow H. R. Radiometric assays for glycerol, glucose, and glycogen. Anal Biochem. 1989 Jul;180(1):11–16. doi: 10.1016/0003-2697(89)90081-x. [DOI] [PubMed] [Google Scholar]
- Castan I., Valet P., Larrouy D., Voisin T., Remaury A., Daviaud D., Laburthe M., Lafontan M. Distribution of PYY receptors in human fat cells: an antilipolytic system alongside the alpha 2-adrenergic system. Am J Physiol. 1993 Jul;265(1 Pt 1):E74–E80. doi: 10.1152/ajpendo.1993.265.1.E74. [DOI] [PubMed] [Google Scholar]
- Coppack S. W., Jensen M. D., Miles J. M. In vivo regulation of lipolysis in humans. J Lipid Res. 1994 Feb;35(2):177–193. [PubMed] [Google Scholar]
- Dolan J. A., Muenkel H. A., Burns M. G., Pellegrino S. M., Fraser C. M., Pietri F., Strosberg A. D., Largis E. E., Dutia M. D., Bloom J. D. Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther. 1994 Jun;269(3):1000–1006. [PubMed] [Google Scholar]
- Durnin J. V., Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974 Jul;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Enocksson S., Shimizu M., Lönnqvist F., Nordenström J., Arner P. Demonstration of an in vivo functional beta 3-adrenoceptor in man. J Clin Invest. 1995 May;95(5):2239–2245. doi: 10.1172/JCI117914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitzky J., Lafontan M., Nordenström J., Arner P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest. 1993 May;91(5):1997–2003. doi: 10.1172/JCI116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobino J. P. Beta 3-adrenoceptor: an update. Eur J Endocrinol. 1995 Apr;132(4):377–385. doi: 10.1530/eje.0.1320377. [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Rao D. D. Rodent and human beta 3-adrenergic receptor genes contain an intron within the protein-coding block. Mol Pharmacol. 1992 Dec;42(6):964–970. [PubMed] [Google Scholar]
- Hellmér J., Marcus C., Sonnenfeld T., Arner P. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992 Jul;75(1):15–20. doi: 10.1210/jcem.75.1.1320047. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Bone D., Ungerstedt U., Jorfeldt L., Henriksson J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci (Lond) 1994 Jan;86(1):15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand. 1991 Nov;143(3):355–356. doi: 10.1111/j.1748-1716.1991.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Hollenga C., Haas M., Deinum J. T., Zaagsma J. Discrepancies in lipolytic activities induced by beta-adrenoceptor agonists in human and rat adipocytes. Horm Metab Res. 1990 Jan;22(1):17–21. doi: 10.1055/s-2007-1004839. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Fowelin J., Smith U., Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol. 1988 Aug;255(2 Pt 1):E218–E220. doi: 10.1152/ajpendo.1988.255.2.E218. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Larsson A., Smith U., Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992 May;89(5):1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. A., Smith U., Lönnroth P. Interstitial glycerol concentration measured by microdialysis in two subcutaneous regions in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E918–E922. doi: 10.1152/ajpendo.1990.258.6.E918. [DOI] [PubMed] [Google Scholar]
- Krief S., Lönnqvist F., Raimbault S., Baude B., Van Spronsen A., Arner P., Strosberg A. D., Ricquier D., Emorine L. J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993 Jan;91(1):344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M., Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993 Jul;34(7):1057–1091. [PubMed] [Google Scholar]
- Lafontan M. Differential recruitment and differential regulation by physiological amines of fat cell beta-1, beta-2 and beta-3 adrenergic receptors expressed in native fat cells and in transfected cell lines. Cell Signal. 1994 May;6(4):363–392. doi: 10.1016/0898-6568(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Krief S., Strosberg A. D., Nyberg S., Emorine L. J., Arner P. Evidence for a functional beta 3-adrenoceptor in man. Br J Pharmacol. 1993 Nov;110(3):929–936. doi: 10.1111/j.1476-5381.1993.tb13902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist F., Thöme A., Nilsell K., Hoffstedt J., Arner P. A pathogenic role of visceral fat beta 3-adrenoceptors in obesity. J Clin Invest. 1995 Mar;95(3):1109–1116. doi: 10.1172/JCI117758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Mauriège P., Marette A., Atgié C., Bouchard C., Thériault G., Bukowiecki L. K., Marceau P., Biron S., Nadeau A., Després J. P. Regional variation in adipose tissue metabolism of severely obese premenopausal women. J Lipid Res. 1995 Apr;36(4):672–684. [PubMed] [Google Scholar]
- Merlet P., Delforge J., Syrota A., Angevin E., Mazière B., Crouzel C., Valette H., Loisance D., Castaigne A., Randé J. L. Positron emission tomography with 11C CGP-12177 to assess beta-adrenergic receptor concentration in idiopathic dilated cardiomyopathy. Circulation. 1993 Apr;87(4):1169–1178. doi: 10.1161/01.cir.87.4.1169. [DOI] [PubMed] [Google Scholar]
- Mohell N., Dicker A. The beta-adrenergic radioligand [3H]CGP-12177, generally classified as an antagonist, is a thermogenic agonist in brown adipose tissue. Biochem J. 1989 Jul 15;261(2):401–405. doi: 10.1042/bj2610401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Revelli J. P., Muzzin P., Paoloni A., Moinat M., Giacobino J. P. Expression of the beta 3-adrenergic receptor in human white adipose tissue. J Mol Endocrinol. 1993 Apr;10(2):193–197. doi: 10.1677/jme.0.0100193. [DOI] [PubMed] [Google Scholar]
- Rosell S., Belfrage E. Blood circulation in adipose tissue. Physiol Rev. 1979 Oct;59(4):1078–1104. doi: 10.1152/physrev.1979.59.4.1078. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Malbon C. C., Hirsch J., Leibel R. L. Lack of beta 3-adrenergic effect on lipolysis in human subcutaneous adipose tissue. J Clin Endocrinol Metab. 1993 Aug;77(2):352–355. doi: 10.1210/jcem.77.2.8393882. [DOI] [PubMed] [Google Scholar]
- Shen Y. T., Zhang H., Vatner S. F. Peripheral vascular effects of beta-3 adrenergic receptor stimulation in conscious dogs. J Pharmacol Exp Ther. 1994 Jan;268(1):466–473. [PubMed] [Google Scholar]
- Ståhle L., Segersvärd S., Ungerstedt U. A comparison between three methods for estimation of extracellular concentrations of exogenous and endogenous compounds by microdialysis. J Pharmacol Methods. 1991 Mar;25(1):41–52. doi: 10.1016/0160-5402(91)90021-v. [DOI] [PubMed] [Google Scholar]
- Thomas R. F., Liggett S. B. Lack of beta 3-adrenergic receptor mRNA expression in adipose and other metabolic tissues in the adult human. Mol Pharmacol. 1993 Mar;43(3):343–348. [PubMed] [Google Scholar]
- Zaagsma J., Nahorski S. R. Is the adipocyte beta-adrenoceptor a prototype for the recently cloned atypical 'beta 3-adrenoceptor'? Trends Pharmacol Sci. 1990 Jan;11(1):3–7. doi: 10.1016/0165-6147(90)90032-4. [DOI] [PubMed] [Google Scholar]


