Abstract
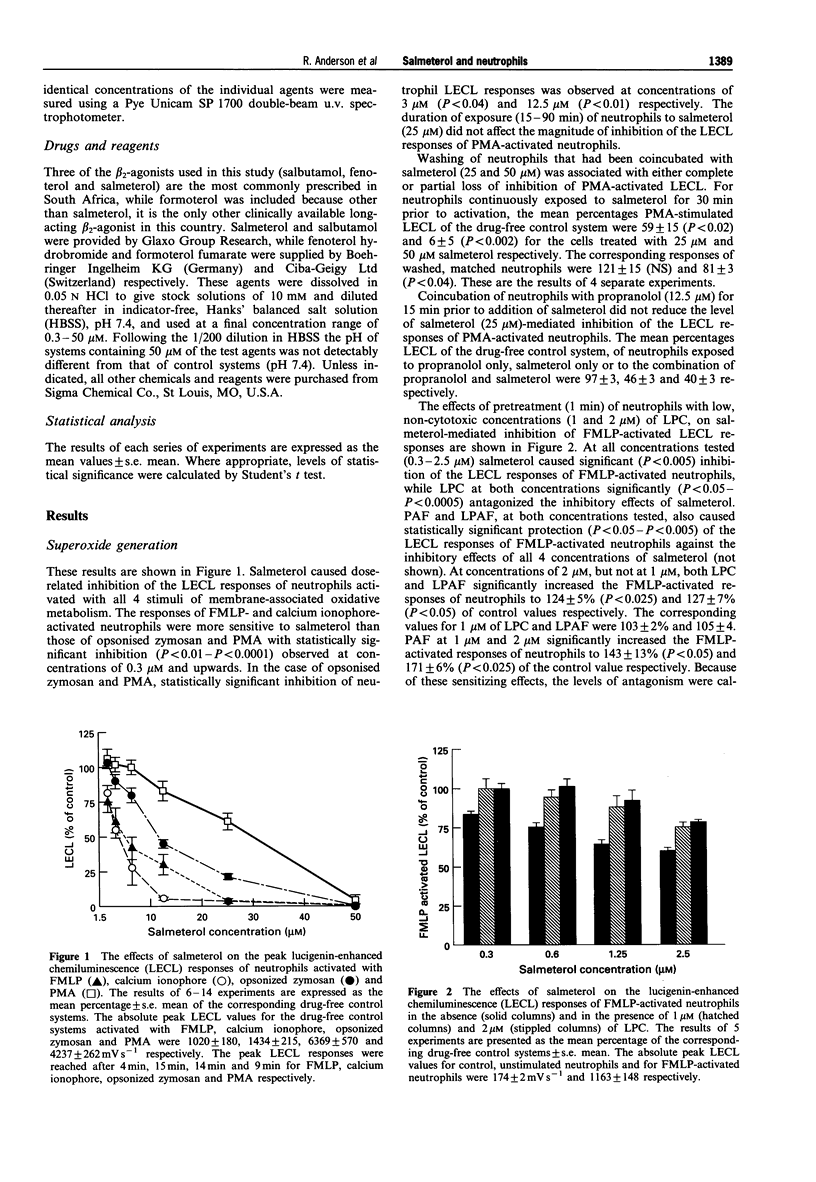
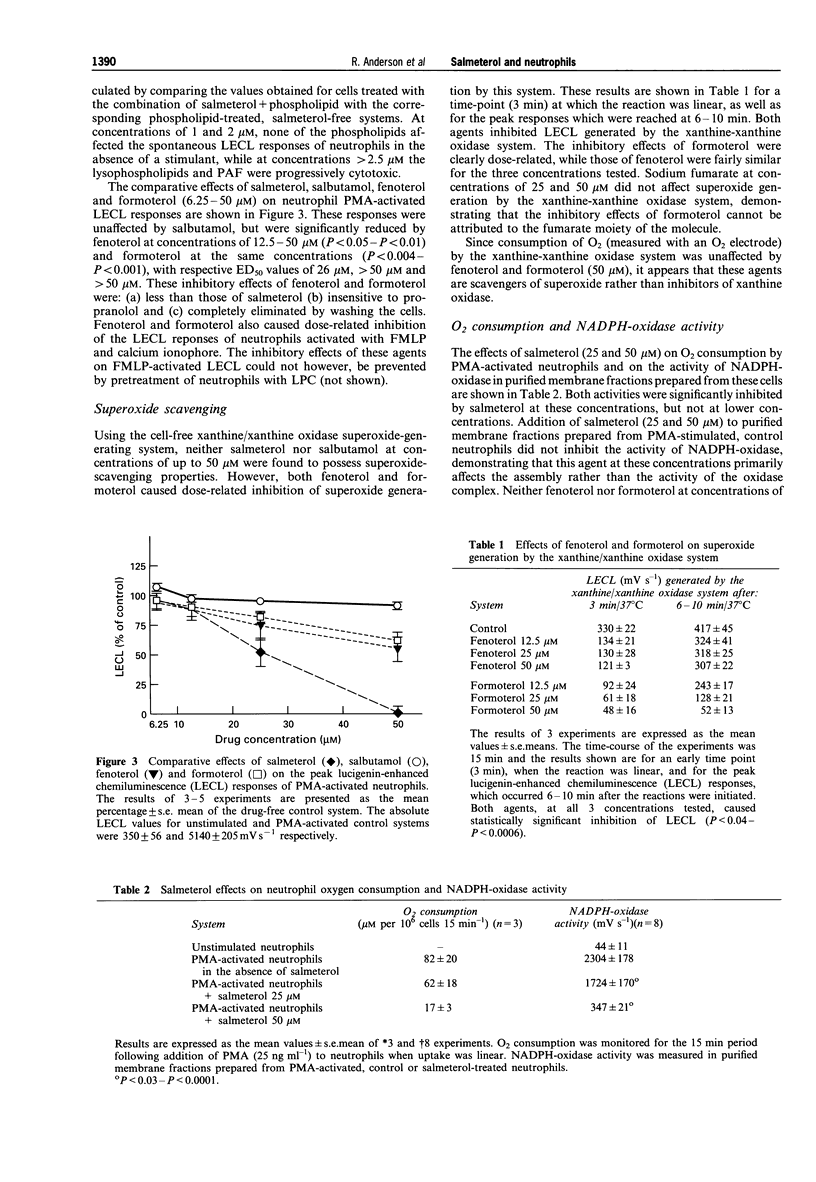
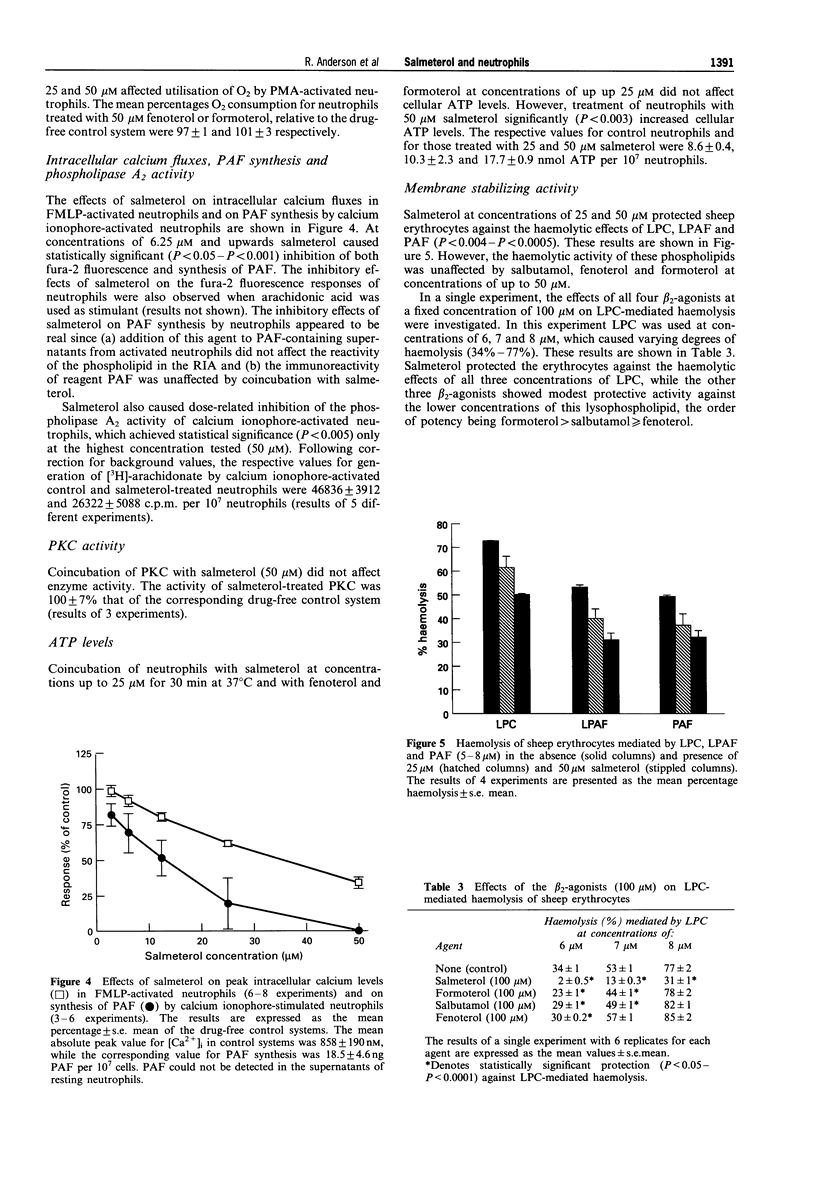
1. We have investigated the effects of salmeterol (0.3-50 microM) on several pro-inflammatory activities of human neutrophils in vitro. 2. Oxidant production by FMLP- and calcium ionophore (A23187)-activated neutrophils was particularly sensitive to inhibition by low concentrations (0.3-3 microM) of salmeterol, while the responses of phorbol myristate acetate- and opsonised zymosan-stimulated cells were affected only by higher concentrations (3-50 microM) of the drug. At these concentrations salmeterol is not cytotoxic, nor does it act as a scavenger of superoxide. 3. These anti-oxidative interactions of salmeterol with neutrophils were insensitive to propranolol but could be eliminated by washing the cells, or by pretreatment with low concentrations (1-2 microM) of the pro-oxidative, membrane-destabilizing phospholipids, lysophosphatidylcholine (LPC), platelet activating factor (PAF) and lysoPAF (LPAF). 4. At concentrations of 6.25-50 microM salmeterol interfered with several other activities of stimulated neutrophils, including intracellular calcium fluxes, phospholipase A2 activity and synthesis of PAF. 5. In an assay of membrane-stabilizing activity, salmeterol (25 and 50 microM) neutralized the haemolytic action of LPC, PAF and LPAF. 6. Of the other commonly used beta 2-adrenoceptor agonists, fenoterol, and formoterol, but not salbutamol, caused moderate inhibition of neutrophil oxidant generation by a superoxide-scavenging mechanism. However, unlike salmeterol, these agents possessed only weak membrane stabilizing properties. 7. We conclude that salmeterol antagonizes the pro-inflammatory, pro-oxidative activity of several bioactive lipids implicated in the pathogenesis of bronchial asthma, by a mechanism related to the membrane-stabilizing, rather than to the beta 2-agonist properties of this agent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advenier C., Qian Y., Koune J. D., Molimard M., Candenas M. L., Naline E. Formoterol and salbutamol inhibit bradykinin- and histamine-induced airway microvascular leakage in guinea-pig. Br J Pharmacol. 1992 Apr;105(4):792–798. doi: 10.1111/j.1476-5381.1992.tb09059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Beyers A. D., Savage J. E., Nel A. E. Apparent involvement of phospholipase A2, but not protein kinase C, in the pro-oxidative interactions of clofazimine with human phagocytes. Biochem Pharmacol. 1988 Dec 15;37(24):4635–4641. doi: 10.1016/0006-2952(88)90332-2. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. New concepts in the pathogenesis of bronchial hyperresponsiveness and asthma. J Allergy Clin Immunol. 1989 Jun;83(6):1013–1026. doi: 10.1016/0091-6749(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Dana R., Malech H. L., Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994 Jan 1;297(Pt 1):217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eda R., Sugiyama H., Hopp R. J., Okada C., Bewtra A. K., Townley R. G. Inhibitory effects of formoterol on platelet-activating factor induced eosinophil chemotaxis and degranulation. Int Arch Allergy Immunol. 1993;102(4):391–398. doi: 10.1159/000236588. [DOI] [PubMed] [Google Scholar]
- Englberger W., Bitter-Suermann D., Hadding U. Influence of lysophospholipids and PAF on the oxidative burst of PMNL. Int J Immunopharmacol. 1987;9(3):275–282. doi: 10.1016/0192-0561(87)90051-8. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Long duration and high potency of antiexudative effects of formoterol in guinea-pig tracheobronchial airways. Am Rev Respir Dis. 1991 Oct;144(4):788–791. doi: 10.1164/ajrccm/144.4.788. [DOI] [PubMed] [Google Scholar]
- Fügner A. Formation of oedema and accumulation of eosinophils in the guinea pig lung. Inhibition by inhaled beta-stimulants. Int Arch Allergy Appl Immunol. 1989;88(1-2):225–227. doi: 10.1159/000234792. [DOI] [PubMed] [Google Scholar]
- Ganbo T., Hisamatsu K., Nakazawa T., Kamijo A., Murakami Y. Platelet activating factor (PAF) effects on ciliary activity of human paranasal sinus mucosa in vitro. Rhinology. 1991 Sep;29(3):231–237. [PubMed] [Google Scholar]
- Ginsburg I., Ward P. A., Varani J. Lysophosphatides enhance superoxide responses of stimulated human neutrophils. Inflammation. 1989 Apr;13(2):163–174. doi: 10.1007/BF00924787. [DOI] [PubMed] [Google Scholar]
- Greening A. P., Ind P. W., Northfield M., Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994 Jul 23;344(8917):219–224. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kanthakumar K., Cundell D. R., Johnson M., Wills P. J., Taylor G. W., Cole P. J., Wilson R. Effect of salmeterol on human nasal epithelial cell ciliary beating: inhibition of the ciliotoxin, pyocyanin. Br J Pharmacol. 1994 Jun;112(2):493–498. doi: 10.1111/j.1476-5381.1994.tb13100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusner D. J., Aucott J. N., Franceschi D., Sarasua M. M., Spagnuolo P. J., King C. H. Protease priming of neutrophil superoxide production. Effects on membrane lipid order and lateral mobility. J Biol Chem. 1991 Sep 5;266(25):16465–16471. [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L. Lysophosphatidylcholine induces mast cell secretion and protein kinase C activation. J Allergy Clin Immunol. 1991 Nov;88(5):721–730. doi: 10.1016/0091-6749(91)90178-q. [DOI] [PubMed] [Google Scholar]
- Mehta D., Gupta S., Gaur S. N., Gangal S. V., Agrawal K. P. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am Rev Respir Dis. 1990 Jul;142(1):157–161. doi: 10.1164/ajrccm/142.1.157. [DOI] [PubMed] [Google Scholar]
- Minkenberg I., Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 1984 Jun 8;71(1):61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., McColl S. R., Caon A. C., Borgeat P. Arachidonic acid-induced mobilization of calcium in human neutrophils: evidence for a multicomponent mechanism of action. Br J Pharmacol. 1989 Jun;97(2):461–468. doi: 10.1111/j.1476-5381.1989.tb11973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen M. M., Moilanen E. K., Nyholm J-E, Koskinen M. O., Karvonen J. I., Metsä-Ketelä T. J., Vapaatalo H. Platelet-activating factor impairs mucociliary transport and increases plasma leukotriene B4 in man. Eur Respir J. 1991 May;4(5):551–560. [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Palmqvist M., Balder B., Löwhagen O., Melander B., Svedmyr N., Wåhlander L. Late asthmatic reaction decreased after pretreatment with salbutamol and formoterol, a new long-acting beta 2-agonist. J Allergy Clin Immunol. 1992 Apr;89(4):844–849. doi: 10.1016/0091-6749(92)90440-d. [DOI] [PubMed] [Google Scholar]
- Ramage L., Blair A. L., Cree I. A., Dhillon D. P. Effect of salmeterol on polymorphonuclear leukocyte (PMNL) chemiluminescence in vitro. J Biolumin Chemilumin. 1993 Sep-Oct;8(5):247–252. doi: 10.1002/bio.1170080504. [DOI] [PubMed] [Google Scholar]
- Twentyman O. P., Finnerty J. P., Harris A., Palmer J., Holgate S. T. Protection against allergen-induced asthma by salmeterol. Lancet. 1990 Dec 1;336(8727):1338–1342. doi: 10.1016/0140-6736(90)92894-n. [DOI] [PubMed] [Google Scholar]
- Whelan C. J., Johnson M., Vardey C. J. Comparison of the anti-inflammatory properties of formoterol, salbutamol and salmeterol in guinea-pig skin and lung. Br J Pharmacol. 1993 Oct;110(2):613–618. doi: 10.1111/j.1476-5381.1993.tb13855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles M. E., Dykens J. A., Wright C. D. Regulation of polymorphonuclear leukocyte membrane fluidity: effect of cytoskeletal modification. J Leukoc Biol. 1994 Aug;56(2):192–199. doi: 10.1002/jlb.56.2.192. [DOI] [PubMed] [Google Scholar]