Abstract
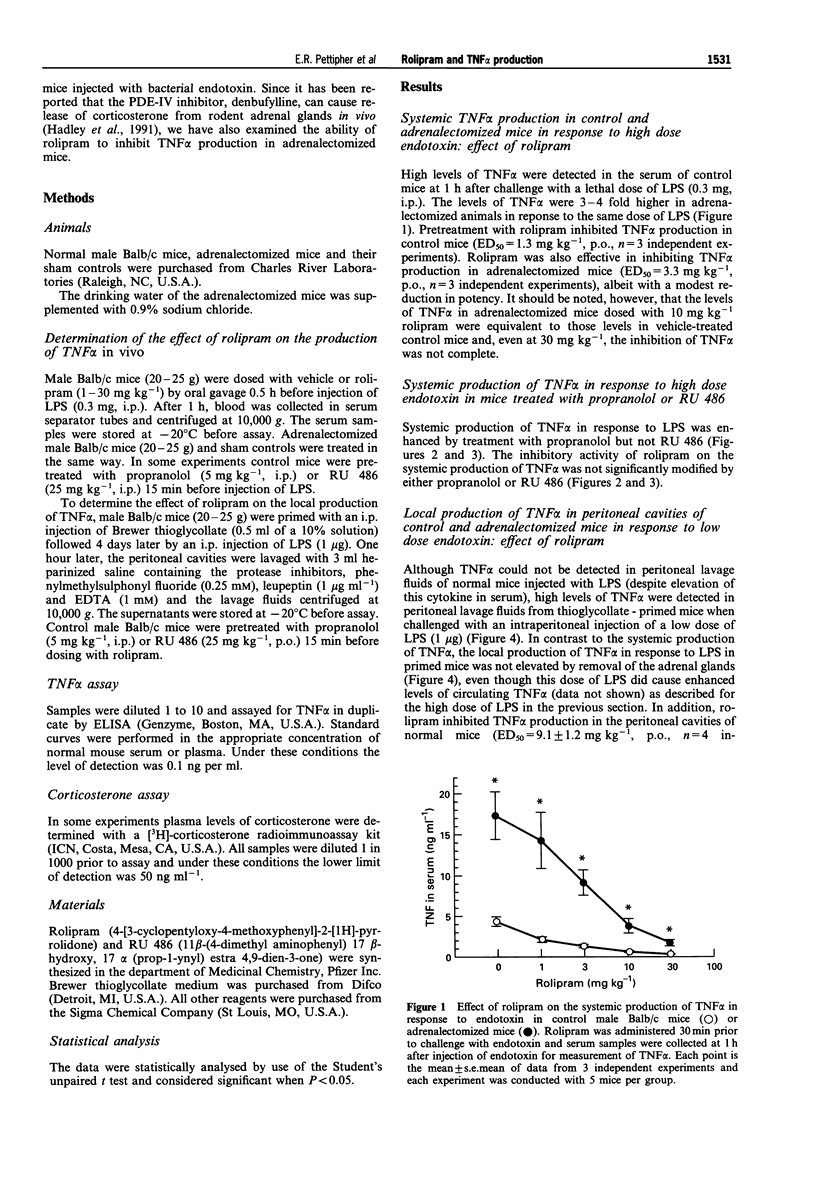
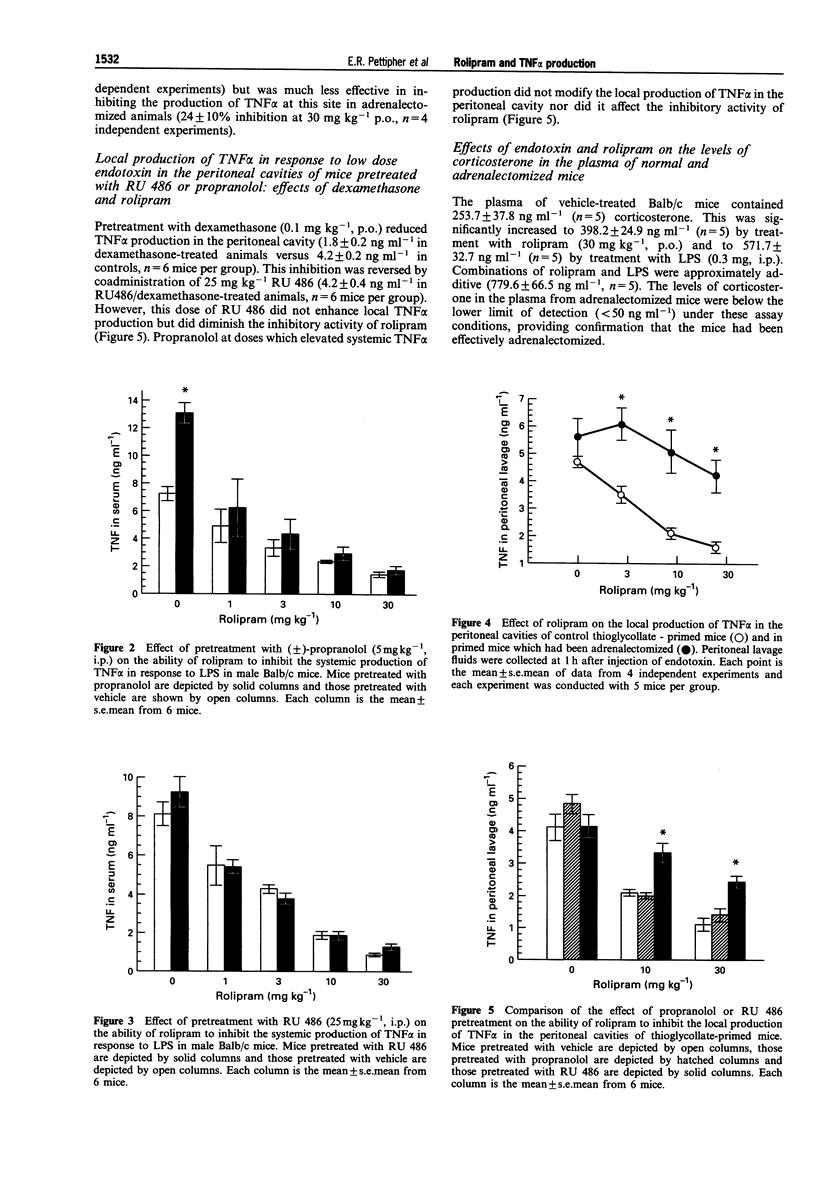
1. The role of adrenal hormones in the regulation of the systemic and local production of tumour necrosis factor (TNF alpha) was examined in male Balb/c mice. 2. Intraperitoneal injection of 0.3 mg E. coli lipopolysaccharide (LPS, 0111:B4) led to high levels of circulating TNF alpha without stimulating TNF alpha production in the peritoneal cavity. Systemic production of TNF alpha in response to LPS was increased in adrenalectomized animals and in normal animals treated with the beta-adrenoceptor antagonist, propranolol. The glucocorticoid antagonist, RU 486, did not modify systemic TNF alpha production. These results indicate that systemic TNF alpha production is regulated by adrenaline but not by corticosterone. 3. When mice were primed with thioglycollate, TNF alpha was produced in the peritoneal cavity in response to low dose LPS (1 micrograms). The levels of TNF alpha in the peritoneal cavity were not enhanced by adrenalectomy or by treatment with either propranolol or RU 486, indicating local production of TNF alpha in the peritoneal cavity is not regulated by adrenaline or corticosterone. 4. The phosphodiesterase type IV (PDE-IV) inhibitor, rolipram, inhibited both the systemic production of TNF alpha in response to high dose endotoxin (ED50 = 1.3 mg kg-1) and the local production of TNF alpha in the peritoneal cavity in response to low dose endotoxin (ED50 = 9.1 mg kg-1). In adrenalectomized mice there was a slight reduction in the ability of rolipram to inhibit the systemic production of TNF alpha (ED50 = 3.3 mg kg-1) while the ability of rolipram to inhibit the local production of TNF alpha in the peritoneal cavity was virtually abolished (24% inhibition at 30 mg kg-1). The glucocorticoid antagonist, RU 486, also reduced the ability of rolipram to inhibit local TNF alpha production while propranolol was without effect. 5. Systemic treatment with rolipram increased the plasma concentrations of corticosterone in normal mice but not in adrenalectomized mice indicating that rolipram can cause adrenal stimulation in vivo. 6. In summary, these data indicate that systemic production of TNF alpha in response to high dose endotoxin is controlled differently from the local production of TNF alpha in response to low dose endotoxin. The systemic production of TNF alpha is regulated by catecholamines, but not by corticosterone, while the local production of TNF alpha in the peritoneal cavity is not regulated by basal levels of either catecholamines or corticosterone. 7. These data also show that the ability of rolipram to inhibit the local production of TNF alpha is dependent on the release of corticosterone from the adrenal glands.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly S., Ferrua B., Fay M., Gougerot-Pocidalo M. A. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990 May;2(3):205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L. D., Layman N. K., Riedl P. E., Cain R. L., Shellhaas J., Evans G. F., Zuckerman S. H. Neuroendocrine regulation of in vivo cytokine production and effects: I. In vivo regulatory networks involving the neuroendocrine system, interleukin-1 and tumor necrosis factor-alpha. J Neuroimmunol. 1989 Sep;24(1-2):143–153. doi: 10.1016/0165-5728(89)90108-2. [DOI] [PubMed] [Google Scholar]
- Dent G., Giembycz M. A., Rabe K. F., Barnes P. J. Inhibition of eosinophil cyclic nucleotide PDE activity and opsonised zymosan-stimulated respiratory burst by 'type IV'-selective PDE inhibitors. Br J Pharmacol. 1991 Jun;103(2):1339–1346. doi: 10.1111/j.1476-5381.1991.tb09790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx B., Taminiau J., Radema S., Stronkhorst A., Wortel C., Tytgat G., van Deventer S. Tumour-necrosis-factor antibody treatment in Crohn's disease. Lancet. 1993 Jul 17;342(8864):173–174. doi: 10.1016/0140-6736(93)91375-v. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Parente L., Persico P., Salmon J. A. A comparison of the acute inflammatory response in adrenalectomised and sham-operated rats. Br J Pharmacol. 1986 Jan;87(1):57–62. doi: 10.1111/j.1476-5381.1986.tb10156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold D. E., Webb E. F., Breton J., White J. R., Marshall P. J., Torphy T. J. Effect of selective phosphodiesterase type IV inhibitor, rolipram, on fluid and cellular phases of inflammatory response. Inflammation. 1993 Jun;17(3):333–344. doi: 10.1007/BF00918994. [DOI] [PubMed] [Google Scholar]
- Hawes A. S., Rock C. S., Keogh C. V., Lowry S. F., Calvano S. E. In vivo effects of the antiglucocorticoid RU 486 on glucocorticoid and cytokine responses to Escherichia coli endotoxin. Infect Immun. 1992 Jul;60(7):2641–2647. doi: 10.1128/iai.60.7.2641-2647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson C. P., Vestal R. E., Sturm R. J., Heaslip R. Effects of selective phosphodiesterase inhibitors on the polymorphonuclear leukocyte respiratory burst. J Allergy Clin Immunol. 1990 Nov;86(5):801–808. doi: 10.1016/s0091-6749(05)80186-1. [DOI] [PubMed] [Google Scholar]
- Parant M., Le Contel C., Parant F., Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991 Aug;10(4):265–271. [PubMed] [Google Scholar]
- Peachell P. T., Undem B. J., Schleimer R. P., MacGlashan D. W., Jr, Lichtenstein L. M., Cieslinski L. B., Torphy T. J. Preliminary identification and role of phosphodiesterase isozymes in human basophils. J Immunol. 1992 Apr 15;148(8):2503–2510. [PubMed] [Google Scholar]
- Peers S. H., Moon D., Flower R. J. Reversal of the anti-inflammatory effects of dexamethasone by the glucocorticoid antagonist RU 38486. Biochem Pharmacol. 1988 Feb 1;37(3):556–557. doi: 10.1016/0006-2952(88)90230-4. [DOI] [PubMed] [Google Scholar]
- Perretti M., Becherucci C., Scapigliati G., Parente L. The effect of adrenalectomy on interleukin-1 release in vitro and in vivo. Br J Pharmacol. 1989 Dec;98(4):1137–1142. doi: 10.1111/j.1476-5381.1989.tb12657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Duncan G. S., Flower R. J., Peers S. H. Serum corticosterone, interleukin-1 and tumour necrosis factor in rat experimental endotoxaemia: comparison between Lewis and Wistar strains. Br J Pharmacol. 1993 Oct;110(2):868–874. doi: 10.1111/j.1476-5381.1993.tb13893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Wimberly D. J. Cyclooxygenase inhibitors enhance tumour necrosis factor production and mortality in murine endotoxic shock. Cytokine. 1994 Sep;6(5):500–503. doi: 10.1016/1043-4666(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Sekut L., Yarnall D., Stimpson S. A., Noel L. S., Bateman-Fite R., Clark R. L., Brackeen M. F., Menius J. A., Jr, Connolly K. M. Anti-inflammatory activity of phosphodiesterase (PDE)-IV inhibitors in acute and chronic models of inflammation. Clin Exp Immunol. 1995 Apr;100(1):126–132. doi: 10.1111/j.1365-2249.1995.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler J., Wachtel H., Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993 Apr;15(3):409–413. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Simpson P. J., Schelm J. A., Smallwood J. K., Clay M. P., Lindstrom T. D. Inhibition of granulocyte cAMP-phosphodiesterase by rolipram in vivo is not sufficient to protect the canine myocardium from reperfusion injury. J Cardiovasc Pharmacol. 1992 Jun;19(6):987–995. doi: 10.1097/00005344-199206000-00022. [DOI] [PubMed] [Google Scholar]
- Sommer N., Löschmann P. A., Northoff G. H., Weller M., Steinbrecher A., Steinbach J. P., Lichtenfels R., Meyermann R., Riethmüller A., Fontana A. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995 Mar;1(3):244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Turner C. R., Esser K. M., Wheeldon E. B. Therapeutic intervention in a rat model of ARDS: IV. Phosphodiesterase IV inhibition. Circ Shock. 1993 Mar;39(3):237–245. [PubMed] [Google Scholar]



