Abstract
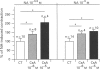
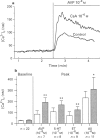
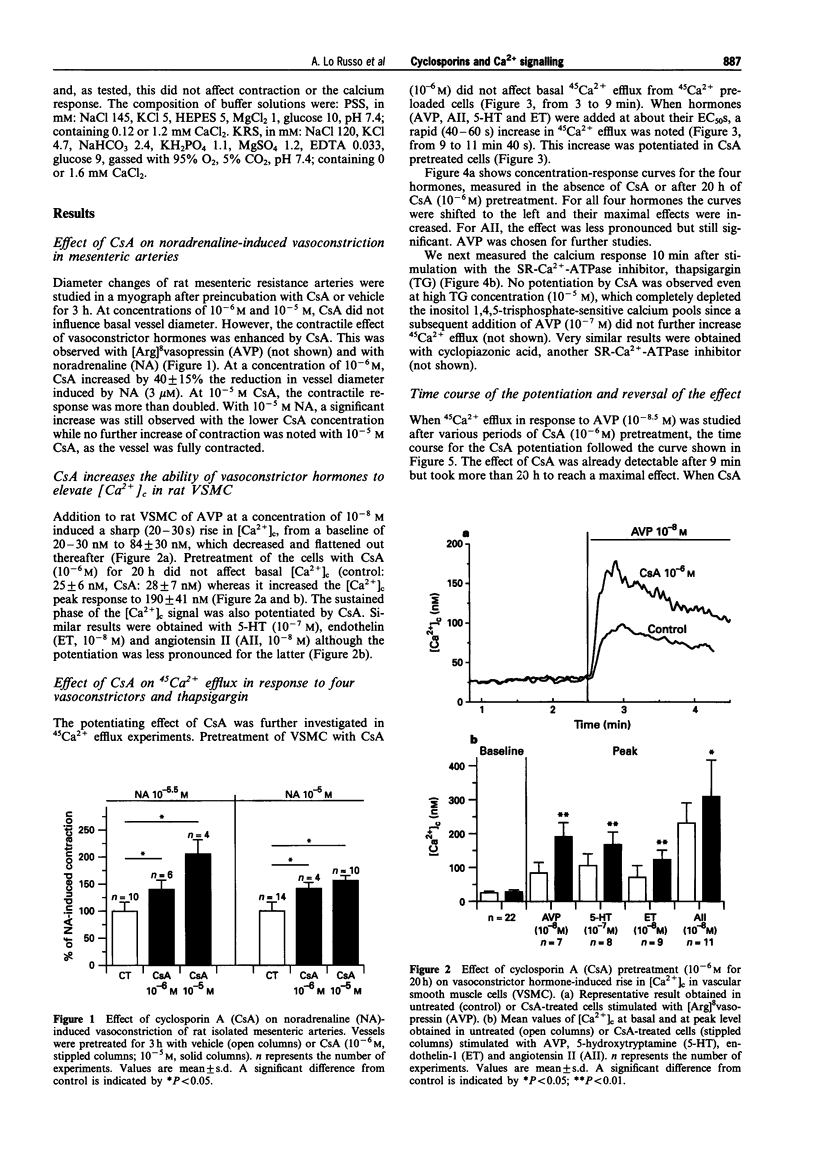
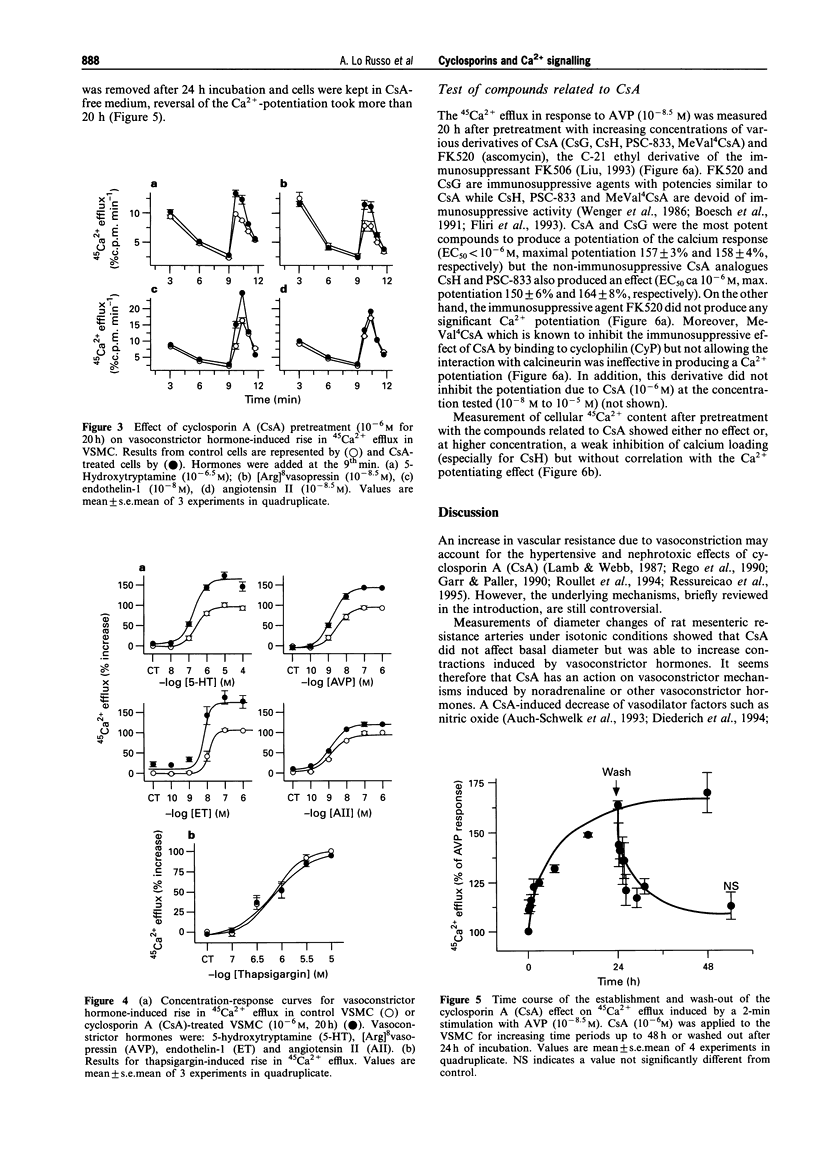
1. The full therapeutic potential of the main immunosuppressive drug, cyclosporin A (CsA), is limited because of its side effects, namely nephrotoxicity and hypertension. Several lines of evidence suggest that the origin of both side effects could be CsA-induced vasoconstriction. However, the underlying molecular mechanisms are not well understood. 2. Diameter measurements of rat isolated mesenteric arteries showed an increase in noradrenaline- and [Arg]8vasopressin-induced vasoconstriction when arteries were pretreated with CsA. 3. Measurements in cultured vascular smooth muscle cells (VSMC) of either cytosolic calcium concentration or of 45Ca2+ efflux showed that CsA potentiated the calcium influx to several vasoconstrictor hormones: [Arg]8vasopressin, angiotensin II, endothelin-1 and 5-hydroxytryptamine. On the other hand, 45Ca2+ efflux in response to thapsigargin, which depletes calcium from intracellular pools, was not potentiated by CsA. 45Ca2+ uptake was not altered by CsA or by any of the analogues tested. 4. Time-course studies in cultured VSMC showed that maximal CsA-induced Ca2+ potentiation occurred after ca. 20 h and this effect was reversed over approximately the next 20 h. 5. To investigate the possible role played by the known intracellular targets of CsA, namely cyclophilin and calcineurin, CsA derivatives with variable potencies with respect to their immunosuppressive activity, were tested on the calcium influx to [Arg]8vasopressin. Derivatives devoid of immunosuppressive activity (cyclosporin H, PSC-833) potentiated calcium signalling, while the potent immunosuppressant, FK520, a close derivative of FK506, and MeVal4CsA, an antagonist of the immunosuppressive effect of CsA did not. The latter compound was unable to reverse the calcium potentiating effect of CsA. 6. Our results show that CsA increases the calcium influx to vasoconstrictor hormones in smooth muscle cells, which presumably increases vasoconstriction. Loading of the intracellular calcium pools appears not to be involved. Experiments with derivatives of CsA and FK520 suggest that interactions with cyclophilins and calcineurin are not the mechanism involved. This indicates, for the first time, that the immunosuppressive activity can be dissociated from the calcium potentiating effect of CsA in vascular smooth muscle.
Full text
PDF




Images in this article
Selected References
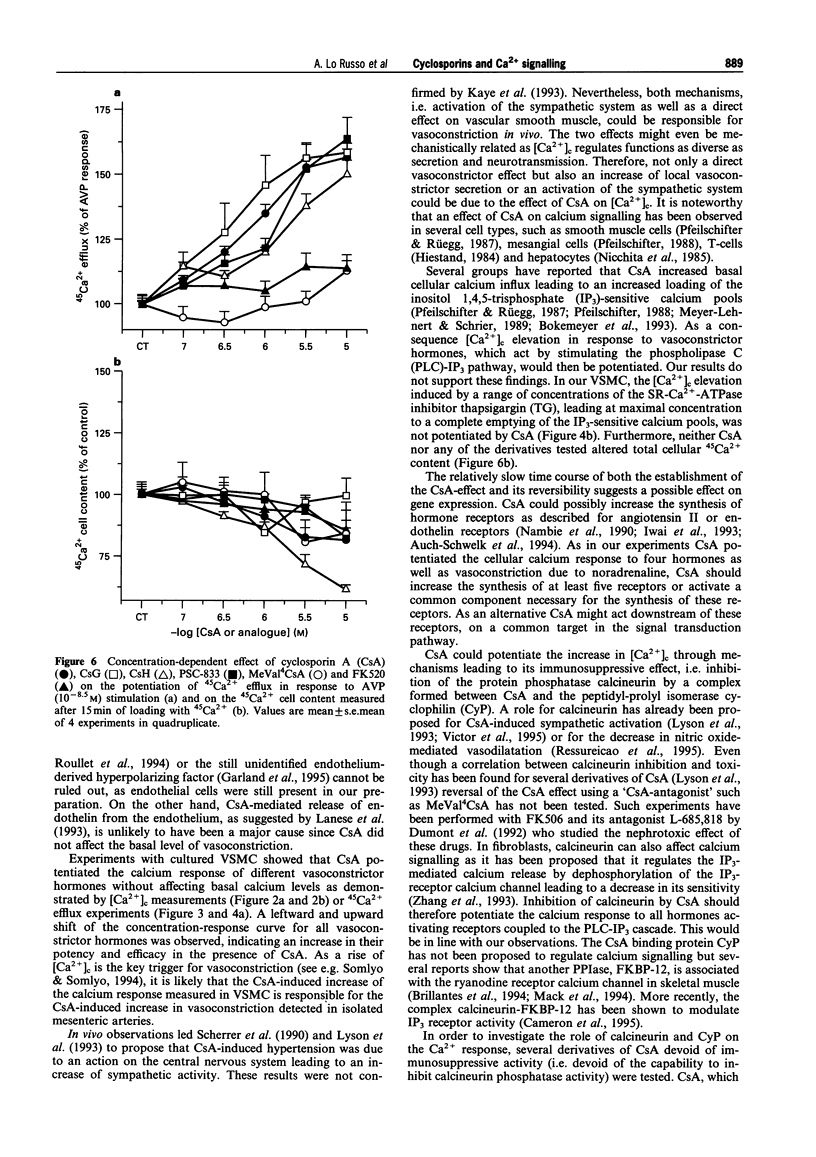
These references are in PubMed. This may not be the complete list of references from this article.
- Auch-Schwelk W., Bossaller C., Götze S., Thelen J., Fleck E. Endothelial and vascular smooth muscle function after chronic treatment with cyclosporin A. J Cardiovasc Pharmacol. 1993 Mar;21(3):435–440. doi: 10.1097/00005344-199303000-00013. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W., Duske E., Hink U., Betz M., Unkelbach M., Fleck E. Vasomotor responses in cyclosporin A-treated rats after chronic angiotensin blockade. Hypertension. 1994 Jun;23(6 Pt 2):832–837. doi: 10.1161/01.hyp.23.6.832. [DOI] [PubMed] [Google Scholar]
- Boesch D., Muller K., Pourtier-Manzanedo A., Loor F. Restoration of daunomycin retention in multidrug-resistant P388 cells by submicromolar concentrations of SDZ PSC 833, a nonimmunosuppressive cyclosporin derivative. Exp Cell Res. 1991 Sep;196(1):26–32. doi: 10.1016/0014-4827(91)90452-z. [DOI] [PubMed] [Google Scholar]
- Bokemeyer D., Kramer H. J., Meyer-Lehnert H. Atrial natriuretic peptide blunts the cellular effects of cyclosporine in smooth muscle. Hypertension. 1993 Feb;21(2):166–172. doi: 10.1161/01.hyp.21.2.166. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994 May 20;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Cameron A. M., Steiner J. P., Roskams A. J., Ali S. M., Ronnett G. V., Snyder S. H. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995 Nov 3;83(3):463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Chiu P. J., Vemulapalli S., Sabin C., Rivelli M., Bernardino V., Sybertz E. J. Sympathoadrenal stimulation, not endothelin, plays a role in acute pressor response to cyclosporine in anesthetized rats. J Pharmacol Exp Ther. 1992 Jun;261(3):994–999. [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Kim G. E., Robinette J. B. Effects of ANG II, ETA, and TxA2 receptor antagonists on cyclosporin A renal vasoconstriction. Am J Physiol. 1994 Sep;267(3 Pt 2):F443–F449. doi: 10.1152/ajprenal.1994.267.3.F443. [DOI] [PubMed] [Google Scholar]
- Copeland K. R., Yatscoff R. W. Comparison of the effects of cyclosporine and its metabolites on the release of prostacyclin and endothelin from mesangial cells. Transplantation. 1992 Mar;53(3):640–645. doi: 10.1097/00007890-199203000-00028. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- Diederich D., Skopec J., Diederich A., Dai F. X. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994 Jun;23(6 Pt 2):957–961. doi: 10.1161/01.hyp.23.6.957. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Kastner C., Iacovone F., Jr, Fischer P. A. Quantitative and temporal analysis of the cellular interaction of FK-506 and rapamycin in T-lymphocytes. J Pharmacol Exp Ther. 1994 Jan;268(1):32–41. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Siekierka J. J., Lin C. S., Harrison R., Sewell T., Kindt V. M., Beattie T. R., Wyvratt M. The immunosuppressive and toxic effects of FK-506 are mechanistically related: pharmacology of a novel antagonist of FK-506 and rapamycin. J Exp Med. 1992 Sep 1;176(3):751–760. doi: 10.1084/jem.176.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993 Jun;24(6):472–495. doi: 10.2165/00003088-199324060-00004. [DOI] [PubMed] [Google Scholar]
- Fliri H., Baumann G., Enz A., Kallen J., Luyten M., Mikol V., Movva R., Quesniaux V., Schreier M., Walkinshaw M. Cyclosporins. Structure-activity relationships. Ann N Y Acad Sci. 1993 Nov 30;696:47–53. [PubMed] [Google Scholar]
- Gallego M. J., García Villalón A. L., López Farre A. J., García J. L., Garrón M. P., Casado S., Hernando L., Caramelo C. A. Mechanisms of the endothelial toxicity of cyclosporin A. Role of nitric oxide, cGMP, and Ca2+. Circ Res. 1994 Mar;74(3):477–484. doi: 10.1161/01.res.74.3.477. [DOI] [PubMed] [Google Scholar]
- Garland C. J., Plane F., Kemp B. K., Cocks T. M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995 Jan;16(1):23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Garr M. D., Paller M. S. Cyclosporine augments renal but not systemic vascular reactivity. Am J Physiol. 1990 Jan;258(1 Pt 2):F211–F217. doi: 10.1152/ajprenal.1990.258.1.F211. [DOI] [PubMed] [Google Scholar]
- Grieff M., Loertscher R., Shohaib S. A., Stewart D. J. Cyclosporine-induced elevation in circulating endothelin-1 in patients with solid-organ transplants. Transplantation. 1993 Oct;56(4):880–884. doi: 10.1097/00007890-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hiestand P. C. Cyclosporin A (Sandimmun) modulates the Ca2+ uptake of mitogen-stimulated lymphocytes. Agents Actions. 1984 Dec;15(5-6):556–561. doi: 10.1007/BF01966774. [DOI] [PubMed] [Google Scholar]
- Iwai J., Kanayama Y., Negoro N., Inoue T., Okamura M., Takeda T. Increased gene expression of angiotensin II type 1A receptor in aortic smooth muscle cells of cyclosporin A-induced hypertensive rats. J Hypertens Suppl. 1993 Dec;11(5):S122–S123. [PubMed] [Google Scholar]
- Julien J., Farge D., Kreft-Jais C., Guyene T. T., Plouin P. F., Houssin D., Carpentier A., Corvol P. Cyclosporine-induced stimulation of the renin-angiotensin system after liver and heart transplantation. Transplantation. 1993 Oct;56(4):885–891. doi: 10.1097/00007890-199310000-00022. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kaye D., Thompson J., Jennings G., Esler M. Cyclosporine therapy after cardiac transplantation causes hypertension and renal vasoconstriction without sympathetic activation. Circulation. 1993 Sep;88(3):1101–1109. doi: 10.1161/01.cir.88.3.1101. [DOI] [PubMed] [Google Scholar]
- Knot H. J., de Ree M. M., Gähwiler B. H., Rüegg U. T. Modulation of electrical activity and of intracellular calcium oscillations of smooth muscle cells by calcium antagonists, agonists, and vasopressin. J Cardiovasc Pharmacol. 1991;18 (Suppl 10):S7–14. [PubMed] [Google Scholar]
- Kremer S., Margolis B., Harper P., Skorecki K. Cyclosporine induced alterations in vasopressin signalling in the glomerular mesangial cell. Clin Invest Med. 1989 Jun;12(3):201–206. [PubMed] [Google Scholar]
- Kunz J., Hall M. N. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem Sci. 1993 Sep;18(9):334–338. doi: 10.1016/0968-0004(93)90069-y. [DOI] [PubMed] [Google Scholar]
- Lamb F. S., Webb R. C. Cyclosporine augments reactivity of isolated blood vessels. Life Sci. 1987 Jun 29;40(26):2571–2578. doi: 10.1016/0024-3205(87)90080-4. [DOI] [PubMed] [Google Scholar]
- Lanese D. M., Conger J. D. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993 May;91(5):2144–2149. doi: 10.1172/JCI116440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. FK506 and ciclosporin: molecular probes for studying intracellular signal transduction. Trends Pharmacol Sci. 1993 May;14(5):182–188. doi: 10.1016/0165-6147(93)90206-y. [DOI] [PubMed] [Google Scholar]
- Locher R., Huss R., Vetter W. Potentiation of vascular smooth muscle cell activity by cyclosporin A. Eur J Clin Pharmacol. 1991;41(4):297–301. doi: 10.1007/BF00314955. [DOI] [PubMed] [Google Scholar]
- Lyson T., Ermel L. D., Belshaw P. J., Alberg D. G., Schreiber S. L., Victor R. G. Cyclosporine- and FK506-induced sympathetic activation correlates with calcineurin-mediated inhibition of T-cell signaling. Circ Res. 1993 Sep;73(3):596–602. doi: 10.1161/01.res.73.3.596. [DOI] [PubMed] [Google Scholar]
- Lyson T., McMullan D. M., Ermel L. D., Morgan B. J., Victor R. G. Mechanism of cyclosporine-induced sympathetic activation and acute hypertension in rats. Hypertension. 1994 May;23(5):667–675. doi: 10.1161/01.hyp.23.5.667. [DOI] [PubMed] [Google Scholar]
- Mack M. M., Molinski T. F., Buck E. D., Pessah I. N. Novel modulators of skeletal muscle FKBP12/calcium channel complex from Ianthella basta. Role of FKBP12 in channel gating. J Biol Chem. 1994 Sep 16;269(37):23236–23249. [PubMed] [Google Scholar]
- Marumo T., Nakaki T., Hishikawa K., Suzuki H., Kato R., Saruta T. Cyclosporin A inhibits nitric oxide synthase induction in vascular smooth muscle cells. Hypertension. 1995 Apr;25(4 Pt 2):764–768. doi: 10.1161/01.hyp.25.4.764. [DOI] [PubMed] [Google Scholar]
- Mason J. Pharmacology of cyclosporine (sandimmune). VII. Pathophysiology and toxicology of cyclosporine in humans and animals. Pharmacol Rev. 1990 Sep;41(3):423–434. [PubMed] [Google Scholar]
- McKeon F. When worlds collide: immunosuppressants meet protein phosphatases. Cell. 1991 Sep 6;66(5):823–826. doi: 10.1016/0092-8674(91)90426-y. [DOI] [PubMed] [Google Scholar]
- Meyer-Lehnert H., Schrier R. W. Potential mechanism of cyclosporine A-induced vascular smooth muscle contraction. Hypertension. 1989 Apr;13(4):352–360. doi: 10.1161/01.hyp.13.4.352. [DOI] [PubMed] [Google Scholar]
- Morgan B. J., Lyson T., Scherrer U., Victor R. G. Cyclosporine causes sympathetically mediated elevations in arterial pressure in rats. Hypertension. 1991 Oct;18(4):458–466. doi: 10.1161/01.hyp.18.4.458. [DOI] [PubMed] [Google Scholar]
- Moss N. G., Powell S. L., Falk R. J. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8222–8226. doi: 10.1073/pnas.82.23.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet C., Benalia H., Carayon A., Bitker M. O., Luciani J., Viars P. Is the vasoconstrictive effect of cyclosporine mediated by endothelin in kidney transplantation? Transplant Proc. 1994 Feb;26(1):277–278. [PubMed] [Google Scholar]
- Müller-Schweinitzer E. Changes in the venous compliance by bradykinin and angiotensin II and its significance for the vascular effects of cyclosporine-A. Naunyn Schmiedebergs Arch Pharmacol. 1988 Dec;338(6):699–703. doi: 10.1007/BF00165637. [DOI] [PubMed] [Google Scholar]
- Nambi P., Pullen M., Contino L. C., Brooks D. P. Upregulation of renal endothelin receptors in rats with cyclosporine A-induced nephrotoxicity. Eur J Pharmacol. 1990 Oct 2;187(1):113–116. doi: 10.1016/0014-2999(90)90346-8. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Kamoun M., Williamson J. R. Cyclosporine augments receptor-mediated cellular Ca2+ fluxes in isolated hepatocytes. J Biol Chem. 1985 Nov 5;260(25):13613–13618. [PubMed] [Google Scholar]
- Pfeilschifter J. Cyclosporin A augments vasoconstrictor-induced rise in intracellular free calcium in rat renal mesangial cells. Biochem Pharmacol. 1988 Nov 1;37(21):4205–4210. doi: 10.1016/0006-2952(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Rüegg U. T. Cyclosporin A augments angiotensin II-stimulated rise in intracellular free calcium in vascular smooth muscle cells. Biochem J. 1987 Dec 15;248(3):883–887. doi: 10.1042/bj2480883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego A., Vargas R., Suarez K. R., Foegh M. L., Ramwell P. W. Mechanism of cyclosporin potentiation of vasoconstriction of the isolated rat mesenteric arterial bed: role of extracellular calcium. J Pharmacol Exp Ther. 1990 Sep;254(3):799–808. [PubMed] [Google Scholar]
- Ressureiço F. A., Ballejo G., Salgado M. C., Ferraz A. S. Effect of cyclosporine administration on vascular reactivity of the isolated mesenteric bed. Transplant Proc. 1995 Apr;27(2):1806–1808. [PubMed] [Google Scholar]
- Roullet J. B., Xue H., McCarron D. A., Holcomb S., Bennett W. M. Vascular mechanisms of cyclosporin-induced hypertension in the rat. J Clin Invest. 1994 May;93(5):2244–2250. doi: 10.1172/JCI117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer U., Vissing S. F., Morgan B. J., Rollins J. A., Tindall R. S., Ring S., Hanson P., Mohanty P. K., Victor R. G. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990 Sep 13;323(11):693–699. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor S. C., Canzanello V. J., Taler S. J., Wilson D. J., Schwartz L. L., Augustine J. E., Raymer J. M., Romero J. C., Wiesner R. H., Krom R. A. Cyclosporine-induced hypertension after transplantation. Mayo Clin Proc. 1994 Dec;69(12):1182–1193. doi: 10.1016/s0025-6196(12)65772-3. [DOI] [PubMed] [Google Scholar]
- Victor R. G., Thomas G. D., Marban E., O'Rourke B. Presynaptic modulation of cortical synaptic activity by calcineurin. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6269–6273. doi: 10.1073/pnas.92.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. X., Zhao H., Muallem S. Ca(2+)-dependent kinase and phosphatase control inositol 1,4,5-trisphosphate-mediated Ca2+ release. Modification by agonist stimulation. J Biol Chem. 1993 May 25;268(15):10997–11001. [PubMed] [Google Scholar]