Abstract
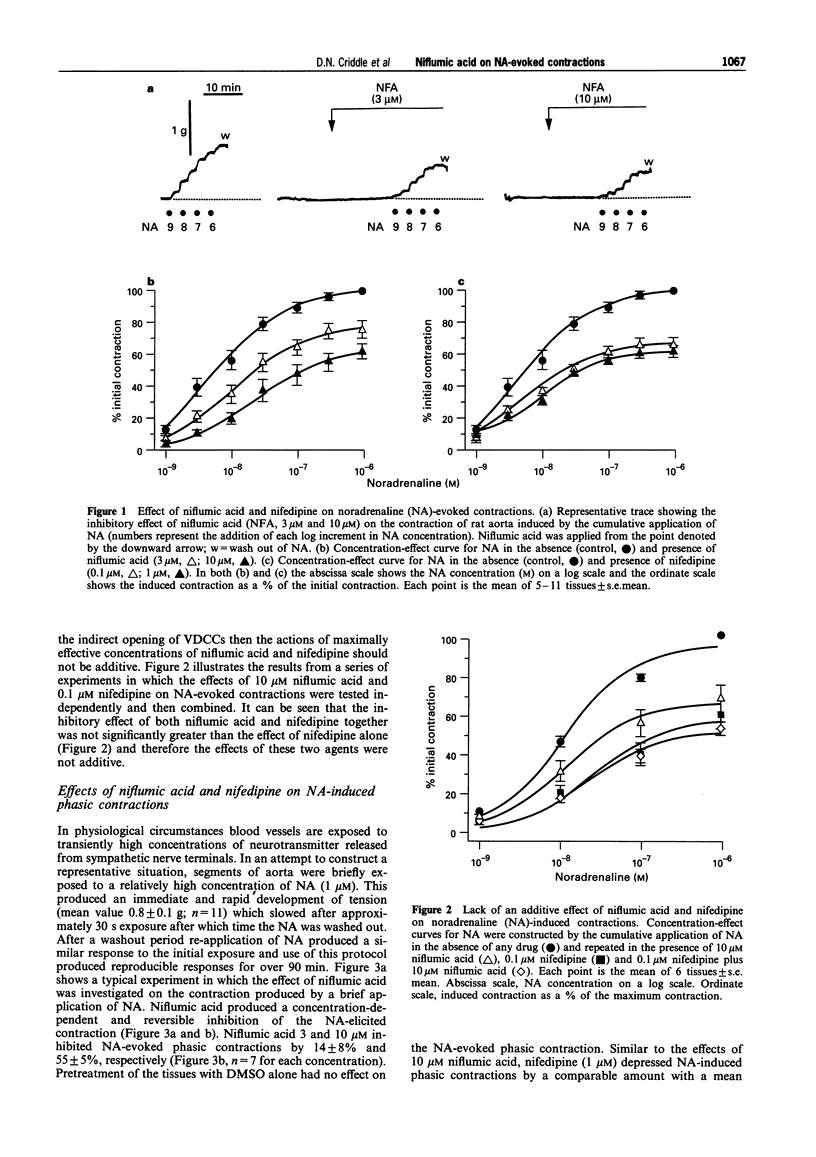
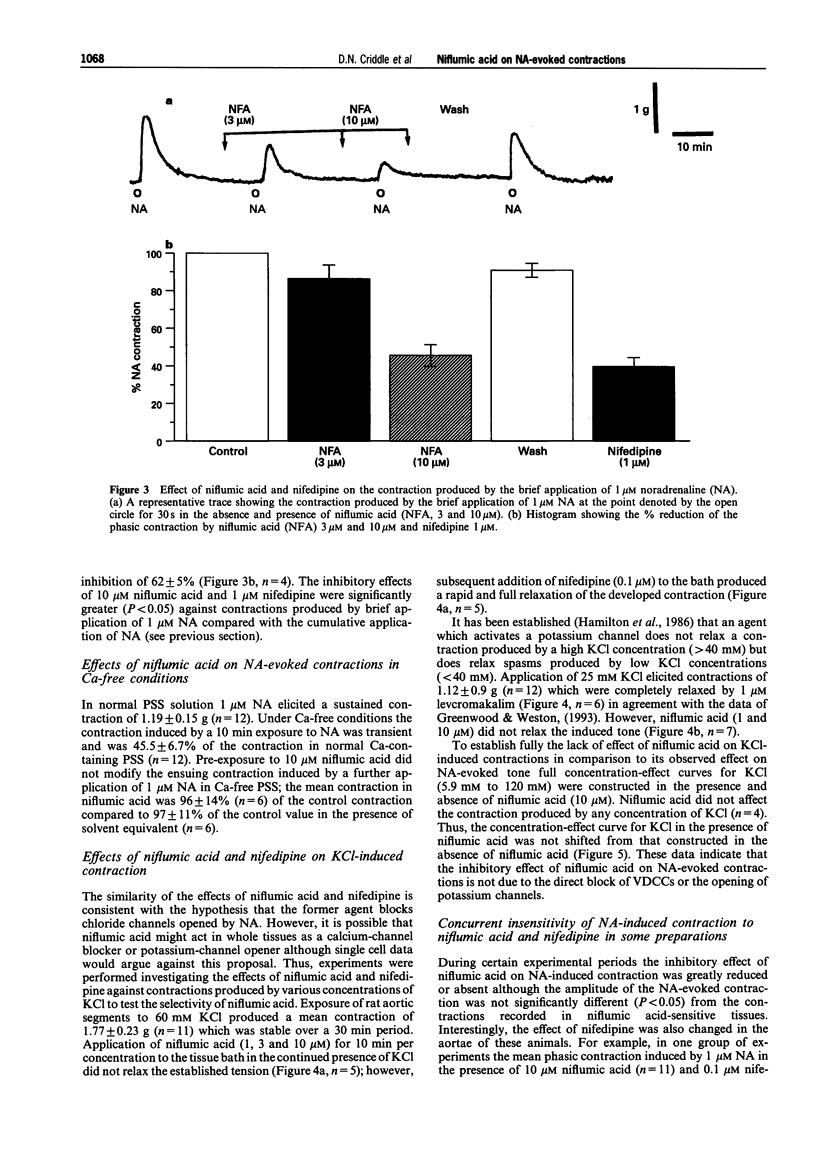
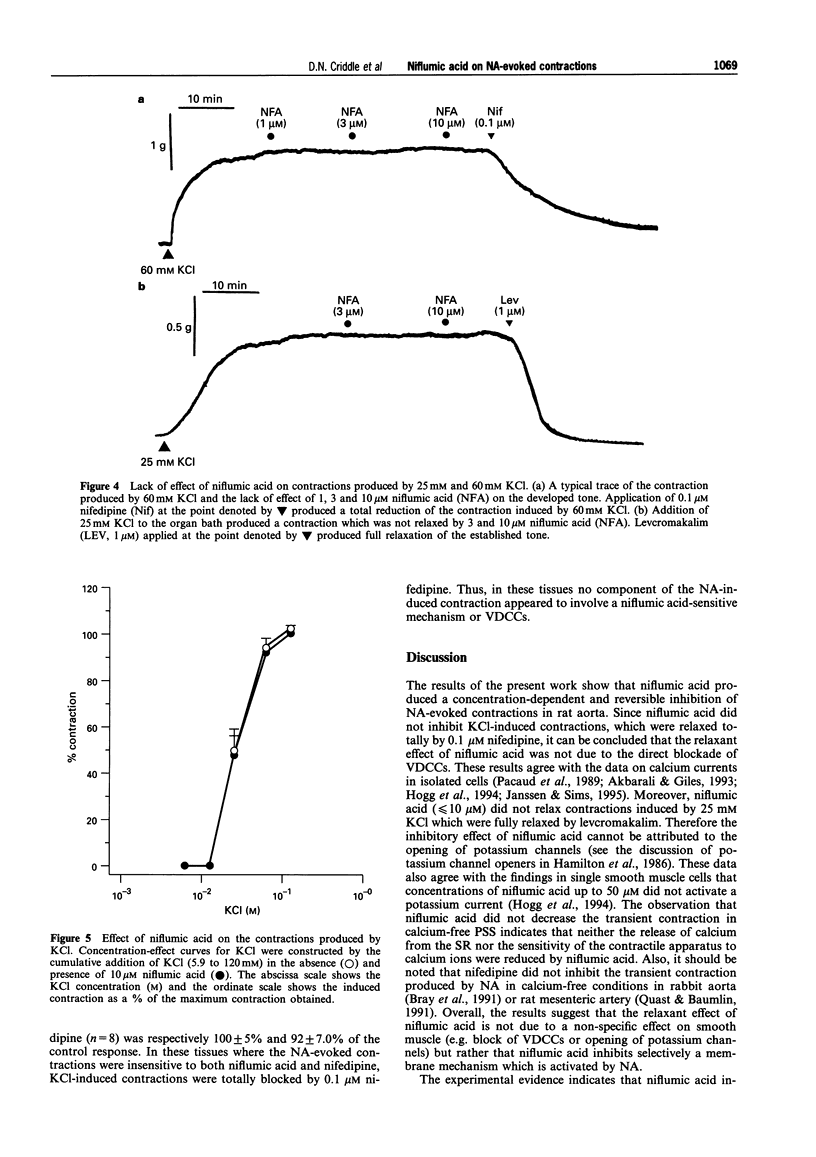
1. The effects of niflumic acid, an inhibitor of calcium-activated chloride channels, were compared with the actions of the calcium channel antagonist nifedipine on noradrenaline-evoked contractions in isolated preparations of the rat aorta. 2. The cumulative concentration-effect curve to noradrenaline (NA) was depressed by both nifedipine and niflumic acid in a reversible and concentration-dependent manner. The degree of inhibition of the maximal contractile response to NA (1 microM) produced by 10 microM niflumic acid (38%) was similar to the effect of 1 microM nifedipine (39%). 3. Contractions to brief applications (30 s) of 1 microM NA were inhibited by 55% and 62% respectively by 10 microM niflumic acid and 1 microM nifedipine. 4. In the presence of 0.1 microM nifedipine, niflumic acid (10 microM) produced no further inhibition of the NA-evoked contractions. Thus, the actions of niflumic acid and nifedipine were not additive. 5. In Ca-free conditions the transient contraction induced by 1 microM NA was not inhibited by niflumic acid (10 microM) and therefore this agent does not reduce the amount of calcium released from the intracellular store or reduce the sensitivity of the contractile apparatus to calcium. 6. Niflumic acid 10 microM did not inhibit the contractions produced by KCl (up to 120 mM) which were totally blocked by nifedipine. Contractions induced by 25 mM KCl were completely inhibited by 1 microM levcromakalim but were unaffected by niflumic acid. 7. It was concluded that niflumic acid produces selective inhibition of a component of NA-evoked contraction which is probably mediated by voltage-gated calcium channels. These data are consistent with a model in which NA stimulates a calcium-activated chloride conductance which leads to the opening of voltage-gated calcium channels to produce contraction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbarali H. I., Giles W. R. Ca2+ and Ca(2+)-activated Cl- currents in rabbit oesophageal smooth muscle. J Physiol. 1993 Jan;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A. Microelectrode study on the ionic mechanisms which contribute to the noradrenaline-induced depolarization in isolated cells of the rabbit portal vein. Br J Pharmacol. 1989 Aug;97(4):1331–1337. doi: 10.1111/j.1476-5381.1989.tb12596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Bray K. M., Weston A. H., Duty S., Newgreen D. T., Longmore J., Edwards G., Brown T. J. Differences between the effects of cromakalim and nifedipine on agonist-induced responses in rabbit aorta. Br J Pharmacol. 1991 Feb;102(2):337–344. doi: 10.1111/j.1476-5381.1991.tb12175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood I. A., Hogg R. C., Large W. A. Effect of frusemide, ethacrynic acid and indanyloxyacetic acid on spontaneous Ca-activated currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995 Jul;115(5):733–738. doi: 10.1111/j.1476-5381.1995.tb14994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood I. A., Weston A. H. Effects of rubidium on responses to potassium channel openers in rat isolated aorta. Br J Pharmacol. 1993 Aug;109(4):925–932. doi: 10.1111/j.1476-5381.1993.tb13709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994 Jul;112(3):977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K., Lin L., Nasjletti A., Goligorsky M. S. Intracellular ramification of endothelin signal. Am J Physiol. 1991 May;260(5 Pt 1):C982–C992. doi: 10.1152/ajpcell.1991.260.5.C982. [DOI] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Ca(2+)-dependent Cl- current in canine tracheal smooth muscle cells. Am J Physiol. 1995 Jul;269(1 Pt 1):C163–C169. doi: 10.1152/ajpcell.1995.269.1.C163. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Endothelin depolarizes myocytes from porcine coronary and human mesenteric arteries through a Ca-activated chloride current. Pflugers Arch. 1991 Mar;418(1-2):168–175. doi: 10.1007/BF00370467. [DOI] [PubMed] [Google Scholar]
- Lamb F. S., Volk K. A., Shibata E. F. Calcium-activated chloride current in rabbit coronary artery myocytes. Circ Res. 1994 Oct;75(4):742–750. doi: 10.1161/01.res.75.4.742. [DOI] [PubMed] [Google Scholar]
- Orallo F., Gil-Longo J., Bardán B., Calleja J. M. Comparison of the effects of hydralazine and nifedipine on contractions and 45Ca influx of rat aorta. J Pharm Pharmacol. 1991 May;43(5):356–359. doi: 10.1111/j.2042-7158.1991.tb06704.x. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Quast U., Baumlin Y. Cromakalim inhibits contractions of the rat isolated mesenteric bed induced by noradrenaline but not caffeine in Ca(2+)-free medium: evidence for interference with receptor-mediated Ca2+ mobilization. Eur J Pharmacol. 1991 Aug 6;200(2-3):239–249. doi: 10.1016/0014-2999(91)90578-e. [DOI] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991 Apr;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]