Abstract
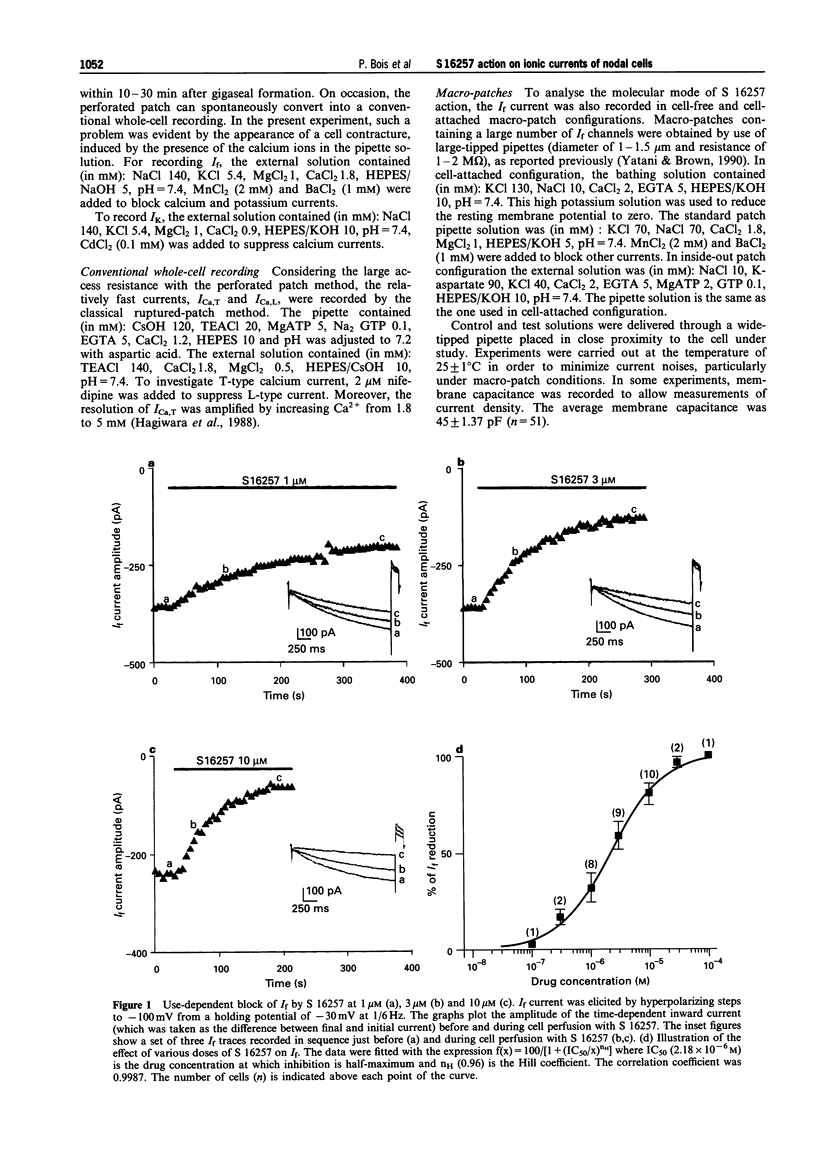
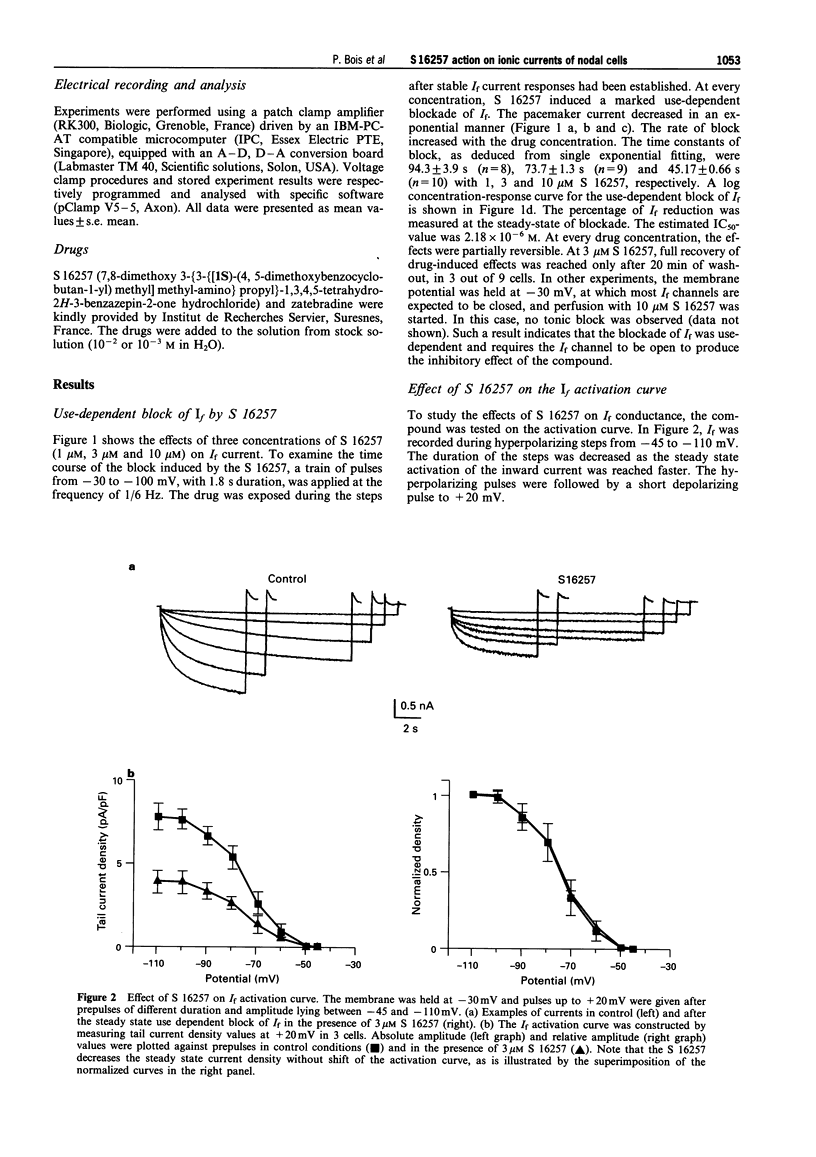
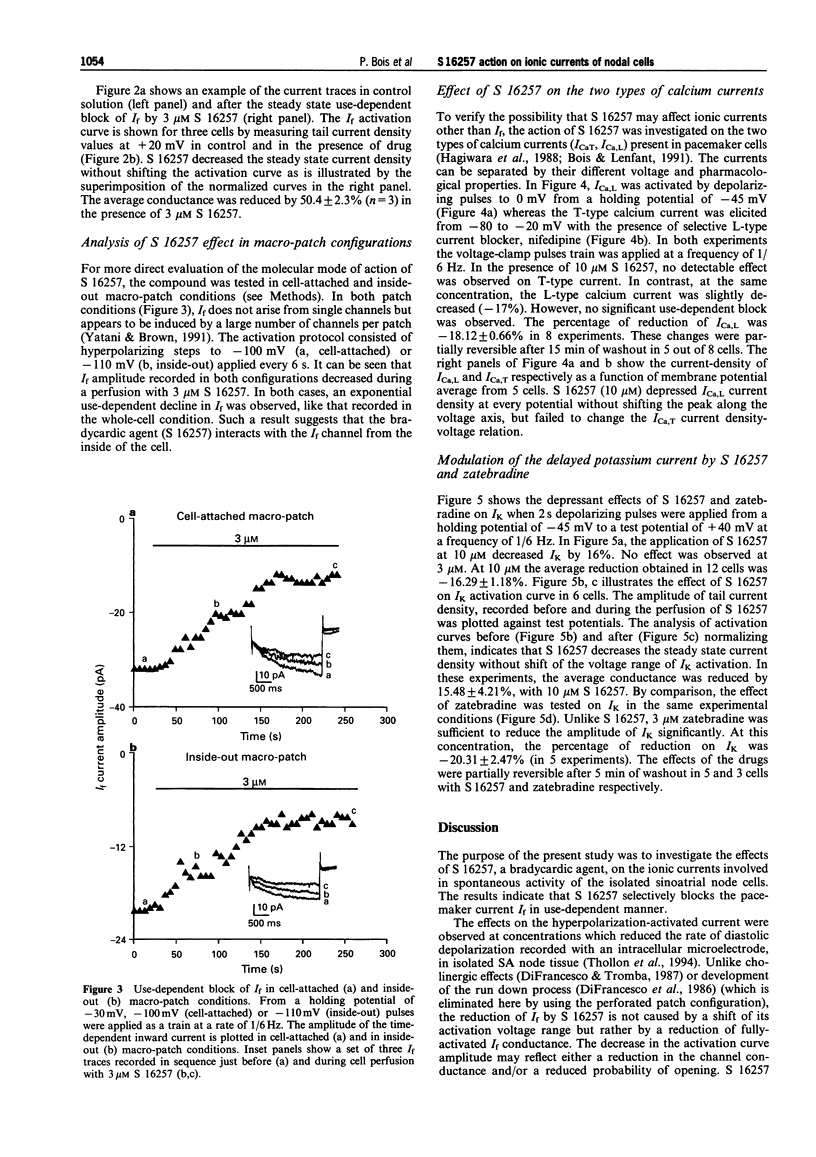
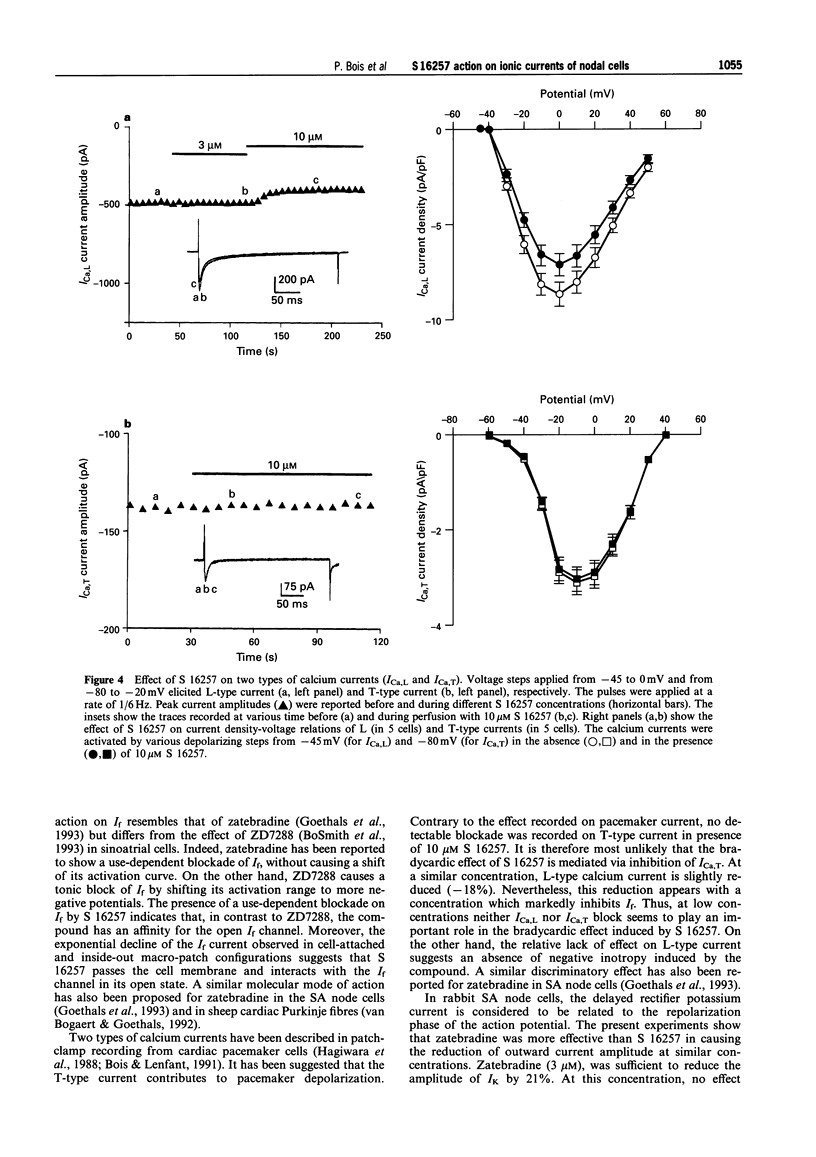
1. The effect of the bradycardic agent S 16257 on the main ionic mechanisms of diastolic depolarization in sinoatrial node cells isolated from rabbit heart, was investigated by the patch-clamp technique in whole-cell and macro-patch recordings. 2. In whole-cell conditions, S 16257 induced a marked exponential use-dependent blockade of the hyperpolarization-activated I(f) current, without shift of the voltage range of its activation curve. The rate of block increased with the drug concentration. The IC50 for the block of I(f) was 2.8 x 10(-6) M. 3. A similar use-dependent decline of I(f) was obtained with 3 microM S 16257, in cell-attached and in inside out macro-patch configurations, suggesting that the bradycardic agent interacts with I(f) channels from the inside of the cell. 4. A high concentration of S 16257 (10 microM) had no detectable effect on T-type calcium current and slightly decreased L-type calcium current (-18.12 +/- 0.66%), without significant use-dependent blockade. 5. S 16257 had no effect on the delayed outward potassium current Ik at 3 microM and slightly decreased it only at high concentrations, -16.3 +/- 1.2% at 10 microM. In contrast, zatebradine, another bradycardic agent, reduced I k by 20.3 +/- 2.5% at 3 microM. 6. In conclusion, S 16257 may lower heart rate without significant negative inotropic action. In comparison with zatebradine, S 16257 had less effect on Ik suggesting less prolongation of repolarization time.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BoSmith R. E., Briggs I., Sturgess N. C. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993 Sep;110(1):343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois P., Lenfant J. Evidence for two types of calcium currents in frog cardiac sinus venosus cells. Pflugers Arch. 1991 Feb;417(6):591–596. doi: 10.1007/BF00372956. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- Goethals M., Raes A., van Bogaert P. P. Use-dependent block of the pacemaker current I(f) in rabbit sinoatrial node cells by zatebradine (UL-FS 49). On the mode of action of sinus node inhibitors. Circulation. 1993 Nov;88(5 Pt 1):2389–2401. doi: 10.1161/01.cir.88.5.2389. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Petit-Jacques J., Bois P., Bescond J., Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993 Apr;423(1-2):21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- Pérez O., Gay P., Franqueza L., Carrón R., Valenzuela C., Delpón E., Tamargo J. Effects of the two enantiomers, S-16257-2 and S-16260-2, of a new bradycardic agent on guinea-pig isolated cardiac preparations. Br J Pharmacol. 1995 Jul;115(5):787–794. doi: 10.1111/j.1476-5381.1995.tb15002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thollon C., Cambarrat C., Vian J., Prost J. F., Peglion J. L., Vilaine J. P. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994 May;112(1):37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert P. P., Goethals M. Blockade of the pacemaker current by intracellular application of UL-FS 49 and UL-AH 99 in sheep cardiac Purkinje fibers. Eur J Pharmacol. 1992 Dec 8;229(1):55–62. doi: 10.1016/0014-2999(92)90285-c. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Regulation of cardiac pacemaker current If in excised membranes from sinoatrial node cells. Am J Physiol. 1990 Jun;258(6 Pt 2):H1947–H1951. doi: 10.1152/ajpheart.1990.258.6.H1947. [DOI] [PubMed] [Google Scholar]


