Abstract
1 The role of nitric oxide (NO) derived from constitutive and inducible nitric oxide synthase (cNOS and iNOS) and its relationship to oxygen-derived free radicals and prostaglandins (PG) was investigated in a carrageenan-induced model of acute hindpaw inflammation. 2 The intraplantar injection of carrageenan elicited an inflammatory response that was characterized by a time-dependent increase in paw oedema, neutrophil infiltration, and increased levels of nitrite/nitrate (NO2-/NO3-) and prostaglandin E2(PGE2) in the paw exudate. 3 Paw oedema was maximal by 6 h and remained elevated for 10 h following carrageenan administration. The non-selective cNOS/iNOS inhibitors, NG-monomethyl-L-arginine (L-NMMA) and NG-nitro-L-arginine methyl ester (L-NAME) given intravenously (30-300 mg kg-1) 1 h before or after carrageenan administration, inhibited paw oedema at all time points. 4 The selective iNOS inhibitors, N-iminoethyl-L-lysine (L-NIL) or aminoguanidine (AG), failed to inhibit carrageenan-induced paw oedema during the first 4 h following carrageenan administration, but inhibited paw oedema at subsequent time points (from 5-10 h). iNOS mRNA was detected between 3 to 10 h following carrageenan administration using ribonuclease protection assays. iNOS protein was first detected 6 h and was maximal 10 h following carrageenan administration as shown by Western blot analysis. Administration of the iNOS inhibitors 5 h after carrageenan (a time point where iNOS was expressed) inhibited paw oedema at all subsequent time points. Infiltrating neutrophils were not the source of iNOS since pretreatment with colchicine (2 mg kg-1) suppressed neutrophil infiltration, but did not inhibit the iNOS mRNA expression or the elevated NO2-/NO3- levels in the paw exudate. 5 Inhibition of paw oedema by the NOS inhibitors was associated with attenuation of both the NO2-/NO3- and PGE2 levels in the paw exudate. These inhibitors also reduced the neutrophil infiltration at the site of inflammation. 6 Recombinant human Cu/Zn superoxide dismutase coupled to polyethyleneglycol (PEGrhSOD; 12 x 10(3) u kg-1), administered intravenously either 30 min prior to or 1 h after carrageenan injection, inhibited paw oedema and neutrophil infiltration, but had no effect on NO2-/NO3- or PGE2 production in the paw exudate. The administration of catalase (40 x 10(3) u kg-1), given intraperitoneally 30 min before carrageenan administration, had no effect on paw oedema. Treatment with desferrioxamine (300 mg kg-1), given subcutaneously 1 h before carrageenan, inhibited paw oedema during the first 2 h after carrageenan administration, but not at later times. 7 These results suggest that the NO produced by cNOS is involved in the development of inflammation at early time points following carrageenan administration and that NO produced by iNOS is involved in the maintenance of the inflammatory response at later time points. The potential interactions of NO with superoxide anion and PG is discussed.
Full text
PDF


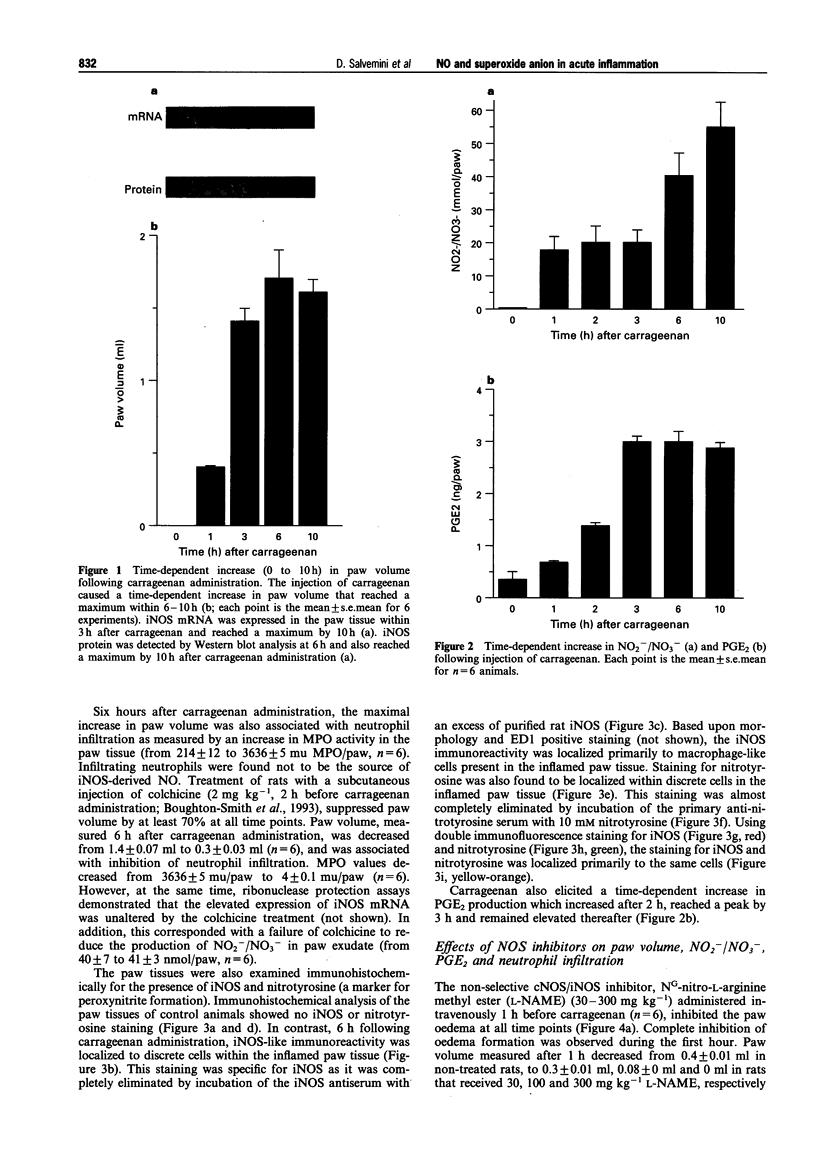

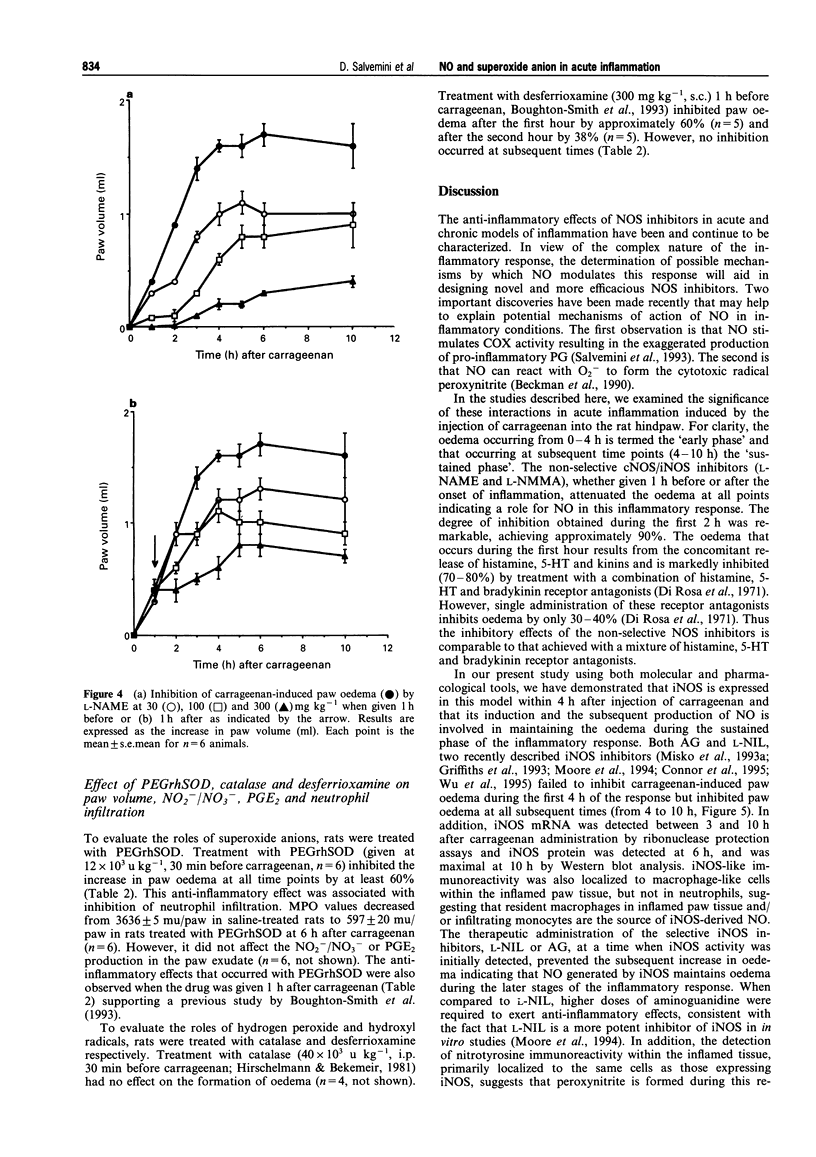
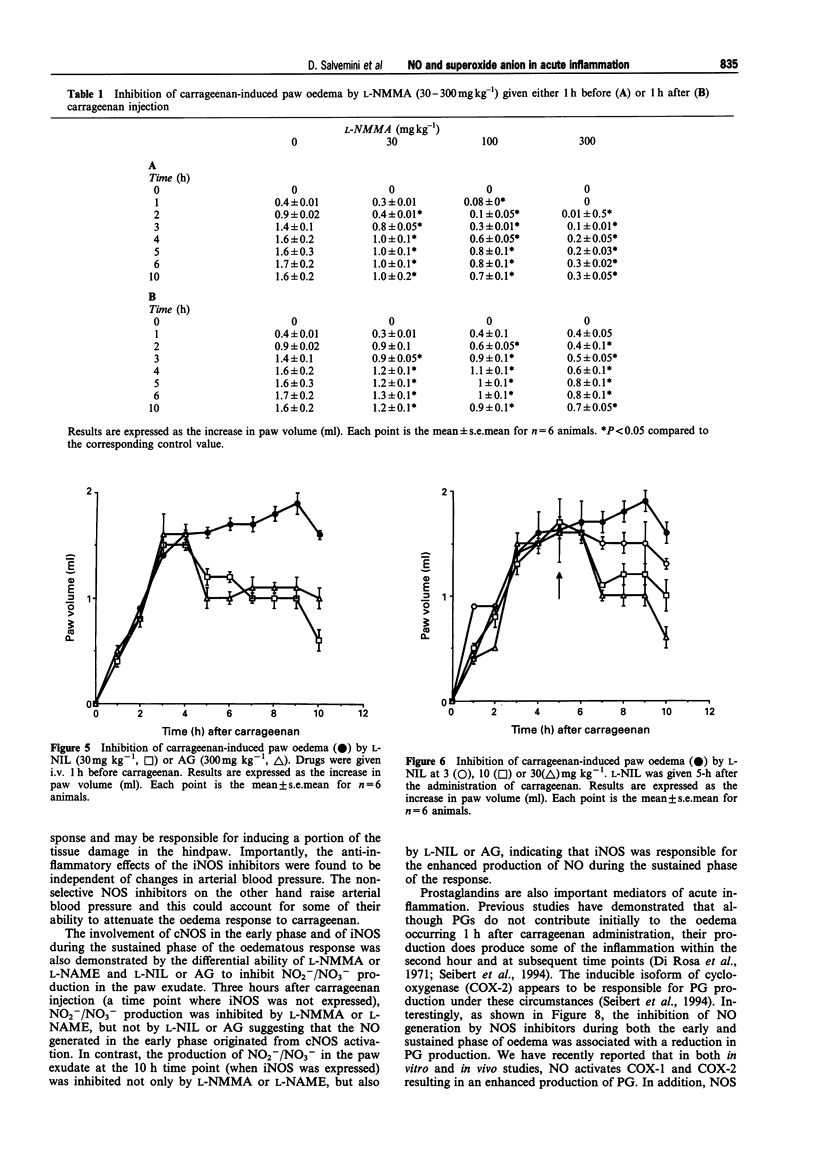
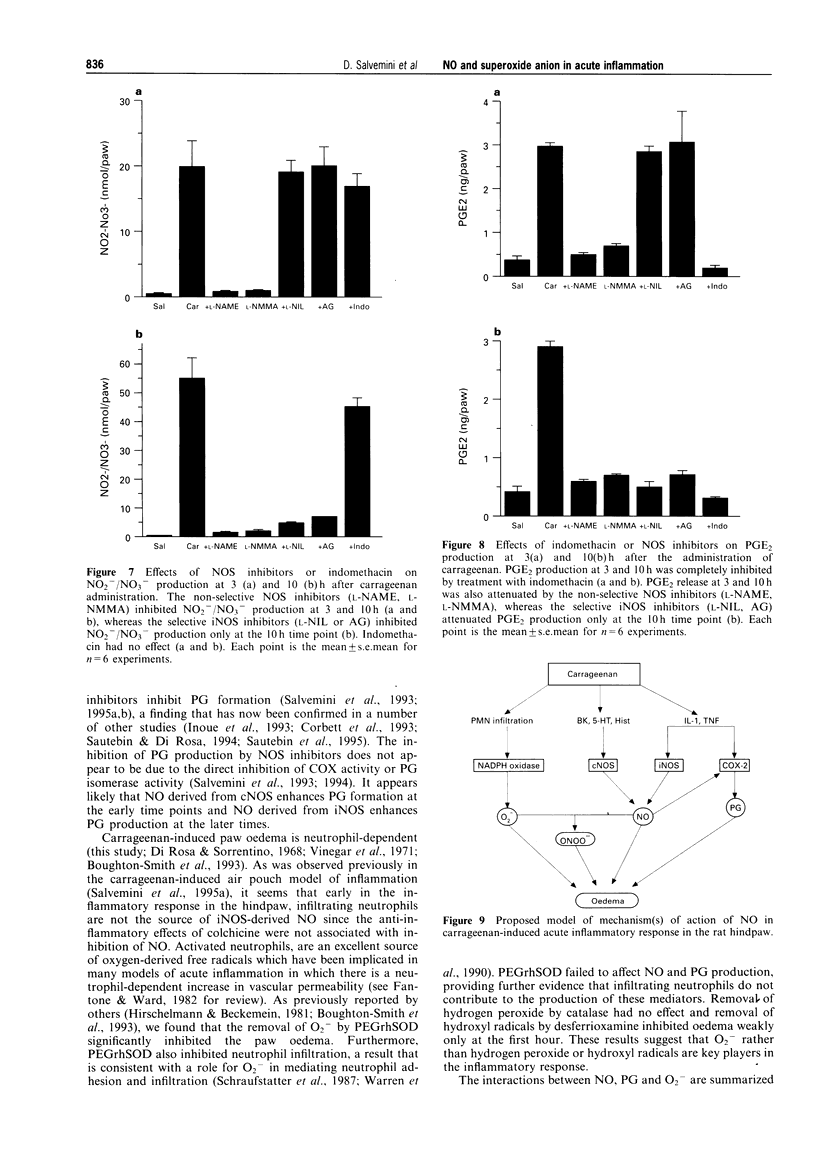


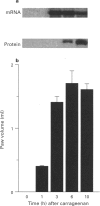
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Deakin A. M., Follenfant R. L., Whittle B. J., Garland L. G. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br J Pharmacol. 1993 Oct;110(2):896–902. doi: 10.1111/j.1476-5381.1993.tb13897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Connor J. R., Manning P. T., Settle S. L., Moore W. M., Jerome G. M., Webber R. K., Tjoeng F. S., Currie M. G. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur J Pharmacol. 1995 Jan 24;273(1-2):15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Kwon G., Turk J., McDaniel M. L. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: activation of cyclooxygenase by nitric oxide. Biochemistry. 1993 Dec 21;32(50):13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- Davidge S. T., Baker P. N., Laughlin M. K., Roberts J. M. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995 Aug;77(2):274–283. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Sorrentino L. The mechanism of the inflammatory effect of carrageenin. Eur J Pharmacol. 1968 Oct;4(3):340–342. doi: 10.1016/0014-2999(68)90103-9. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Willoughby D. A. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971 Apr;23(4):297–298. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. J., Messent M., MacAllister R. J., Evans T. W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschelmann R., Bekemeier H. Effects of catalase, peroxidase, superoxide dismutase and 10 scavengers of oxygen radicals in carrageenin edema and in adjuvant arthritis of rats. Experientia. 1981 Dec 15;37(12):1313–1314. doi: 10.1007/BF01948381. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Ianaro A., O'Donnell C. A., Di Rosa M., Liew F. Y. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology. 1994 Jul;82(3):370–375. [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Fukuo K., Morimoto S., Koh E., Ogihara T. Nitric oxide mediates interleukin-1-induced prostaglandin E2 production by vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 15;194(1):420–424. doi: 10.1006/bbrc.1993.1836. [DOI] [PubMed] [Google Scholar]
- Laight D. W., Lad N., Woodward B., Waterfall J. F. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur J Pharmacol. 1994 Nov 1;292(1):81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Schilling R. J., Salvemini D., Moore W. M., Currie M. G. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993 Oct;214(1):11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore W. M., Webber R. K., Jerome G. M., Tjoeng F. S., Misko T. P., Currie M. G. L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994 Nov 11;37(23):3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Rettori V., Gimeno M., Lyson K., McCann S. M. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., Kirk M., Freeman B. A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994 Oct 21;269(42):26066–26075. [PubMed] [Google Scholar]
- Salvemini D., Manning P. T., Zweifel B. S., Seibert K., Connor J., Currie M. G., Needleman P., Masferrer J. L. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest. 1995 Jul;96(1):301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Misko T. P., Masferrer J. L., Seibert K., Currie M. G., Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Seibert K., Masferrer J. L., Misko T. P., Currie M. G., Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994 May;93(5):1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Settle S. L., Masferrer J. L., Seibert K., Currie M. G., Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol. 1995 Mar;114(6):1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautebin L., Di Rosa M. Nitric oxide modulates prostacyclin biosynthesis in the lung of endotoxin-treated rats. Eur J Pharmacol. 1994 Sep 1;262(1-2):193–196. doi: 10.1016/0014-2999(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Sautebin L., Ialenti A., Ianaro A., Di Rosa M. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br J Pharmacol. 1995 Jan;114(2):323–328. doi: 10.1111/j.1476-5381.1995.tb13230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Jackson J., Cochrane C. C. Oxidant injury of cells. Int J Tissue React. 1987;9(4):317–324. [PubMed] [Google Scholar]
- Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969 Mar;166(1):96–103. [PubMed] [Google Scholar]
- Warren J. B., Coughlan M. L., Williams T. J. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. doi: 10.1111/j.1476-5381.1992.tb14441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Mandel D. M., Johnson K. J., Ward P. A. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med. 1990;8(2):163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Chen S. J., Szabó C., Thiemermann C., Vane J. R. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995 Apr;114(8):1666–1672. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]