Abstract
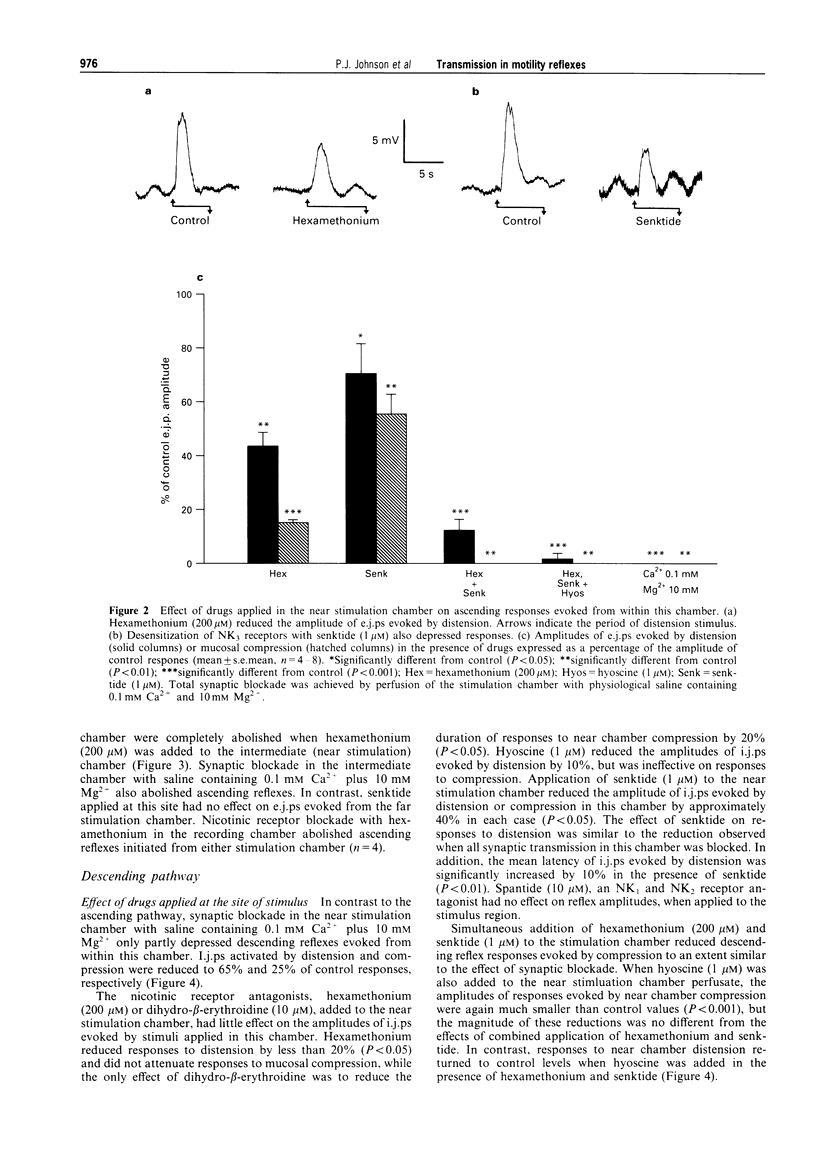
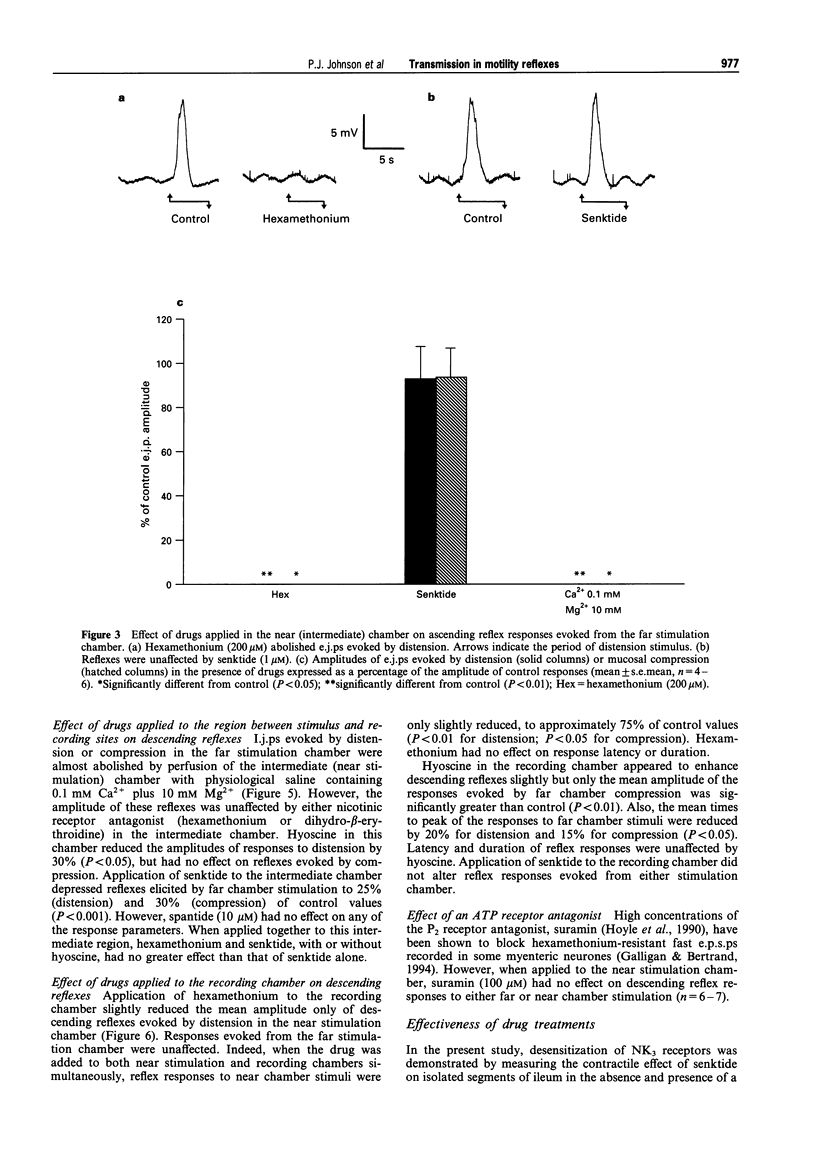
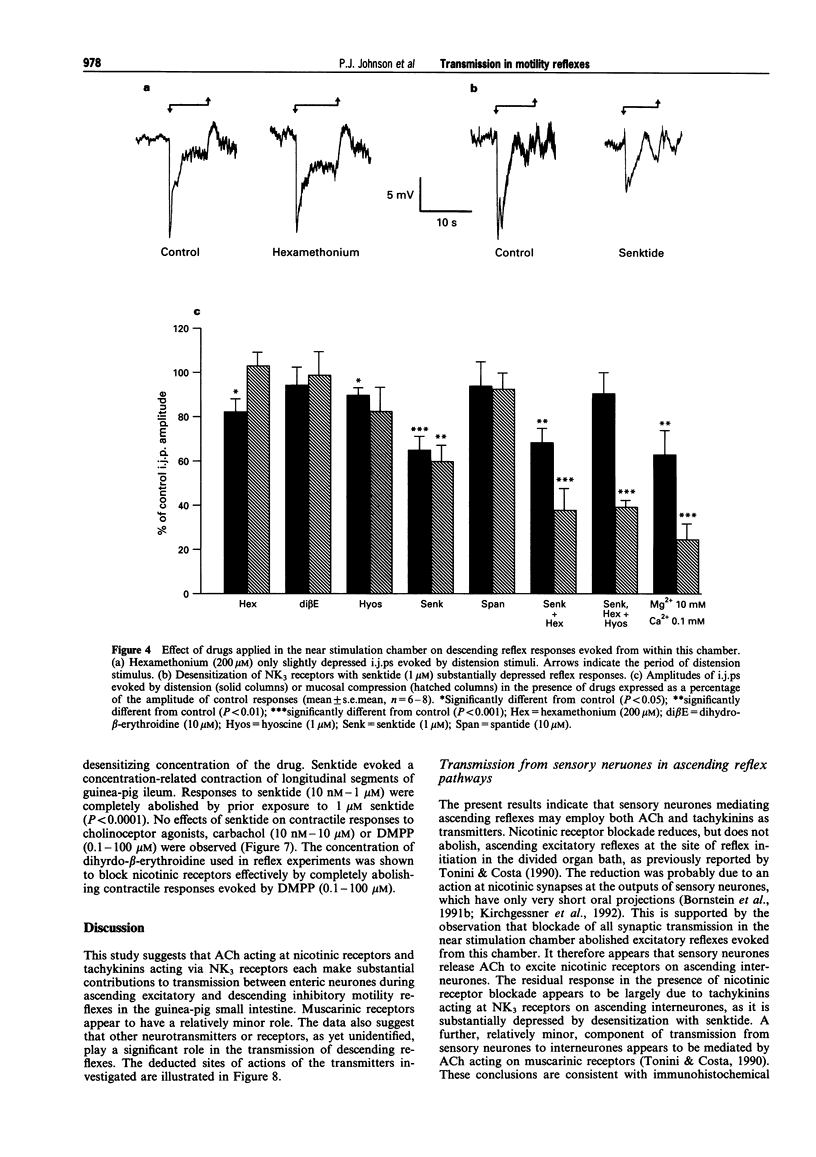
1. The roles of acetylcholine (ACh) and tachykinins in neuro-neuronal transmission during ascending excitatory and descending inhibitory reflexes were studied by recording intracellular reflex responses of the circular muscle to physiological stimuli. Experiments were carried out in opened segments of guinea pig ileum in an organ bath that was partitioned so that three regions could be independently exposed to drugs. 2. Ascending excitatory reflexes evoked by either distension from the serosal side or compression of the mucosa were depressed by 55% and 85%, respectively, in the presence of hexamethonium (200 microM) and by 30% and 45%, respectively, by a desensitizing concentration of the selective NK3 receptor agonist, senktide (1 microM), in the chamber in which reflexes were initiated. Together, hexamethonium and senktide abolished responses to compression. A residual response to distension persisted. This was abolished by hyoscine (1 microM). 3. Hexamethonium (200 microM) abolished ascending reflexes when applied to the region between the stimulus and the recording sites, or to the recording chamber. 4. Descending reflex responses were reduced by 35% by synaptic blockade in the stimulus chamber with physiological saline containing 0.1 mM Ca2+ plus 10 mM Mg2+. Senktide (1 microM) in the stimulus chamber reduced distension reflexes to the same extent as synaptic blockade, whereas hexamethonium (200 microM) and hyoscine (1 microM) depressed responses by less than 20%. Responses to compression were reduced by 40% by senktide alone, while senktide and hexamethonium together reduced responses by 60%, an effect similar to synaptic blockade. Under these conditions, hyoscine in the stimulus chamber restored reflexes evoked by distension, but did not alter those evoked by mucosal compression. 5. Total synaptic blockade in the intermediate chamber, between stimulus and recording sites, reduced descending reflex responses by more than 90%. In contrast, hexamethonium (200 microM) had no effect and hyoscine (1 microM) reduced only the responses to distension (by 30%). Senktide (1 microM) depressed responses to both stimuli by approximately 80%. 6. Application of hexamethonium (200 microM) to the recording chamber depressed descending reflex responses to distension applied in the near stimulation chamber by 15%, but had no effect on responses to compression in the near chamber or to either stimulus applied in the far chamber. 7. Descending reflexes evoked by near chamber stimuli were unaffected by hyoscine (1 microM) or senktide (1 microM) applied to the recording chamber; hyoscine enhanced reflexes evoked by compression in the far chamber by 50%. 8. For the ascending excitatory reflex pathway, it is concluded that transmission from sensory neurones is mediated by ACh acting via both nicotinic and muscarinic receptors, and by tachykinins acting at NK3 receptors. Transmission from ascending interneurones appears to be predominantly via nicotinic receptors. The descending inhibitory pathways are more complex, and while transmission from sensory neurones involves nicotinic, muscarinic and NK3 receptor-dependent components, transmission from descending interneurones to inhibitory motor neurones is neither cholinergic nor due to tachykinins acting via NK3 receptors.
Full text
PDF


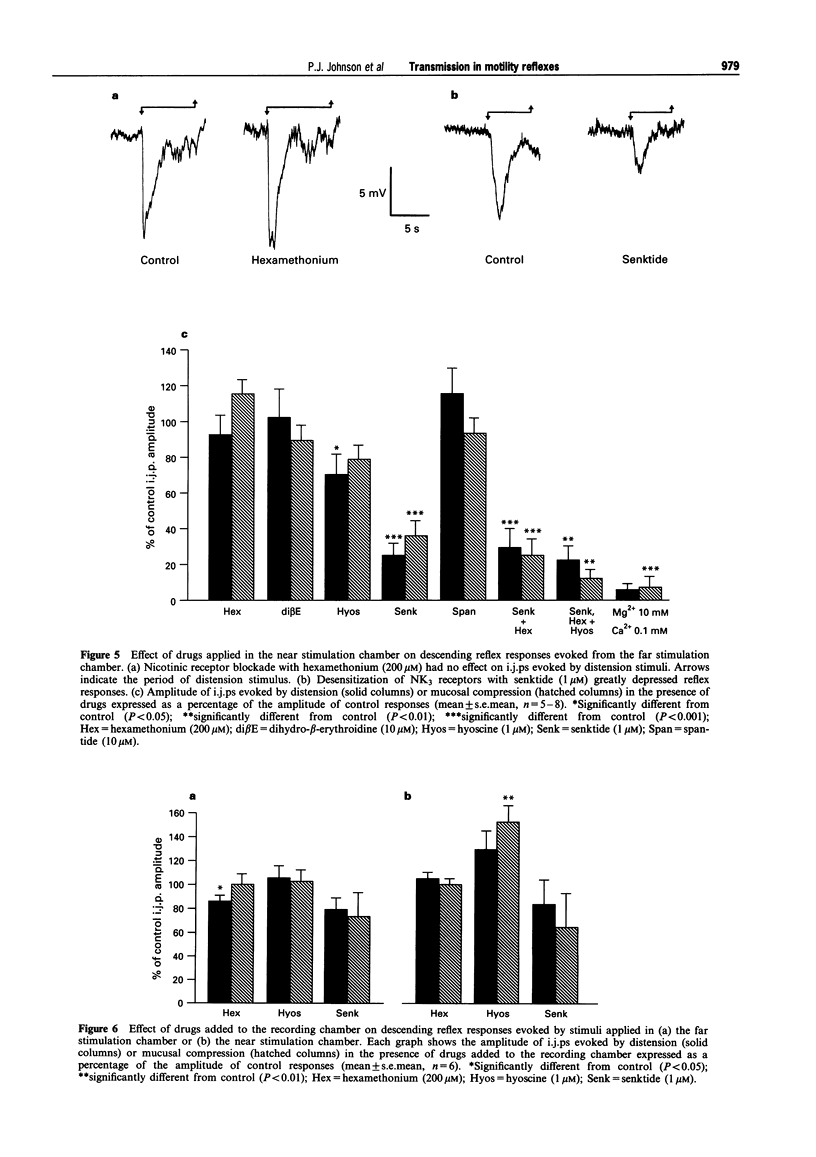
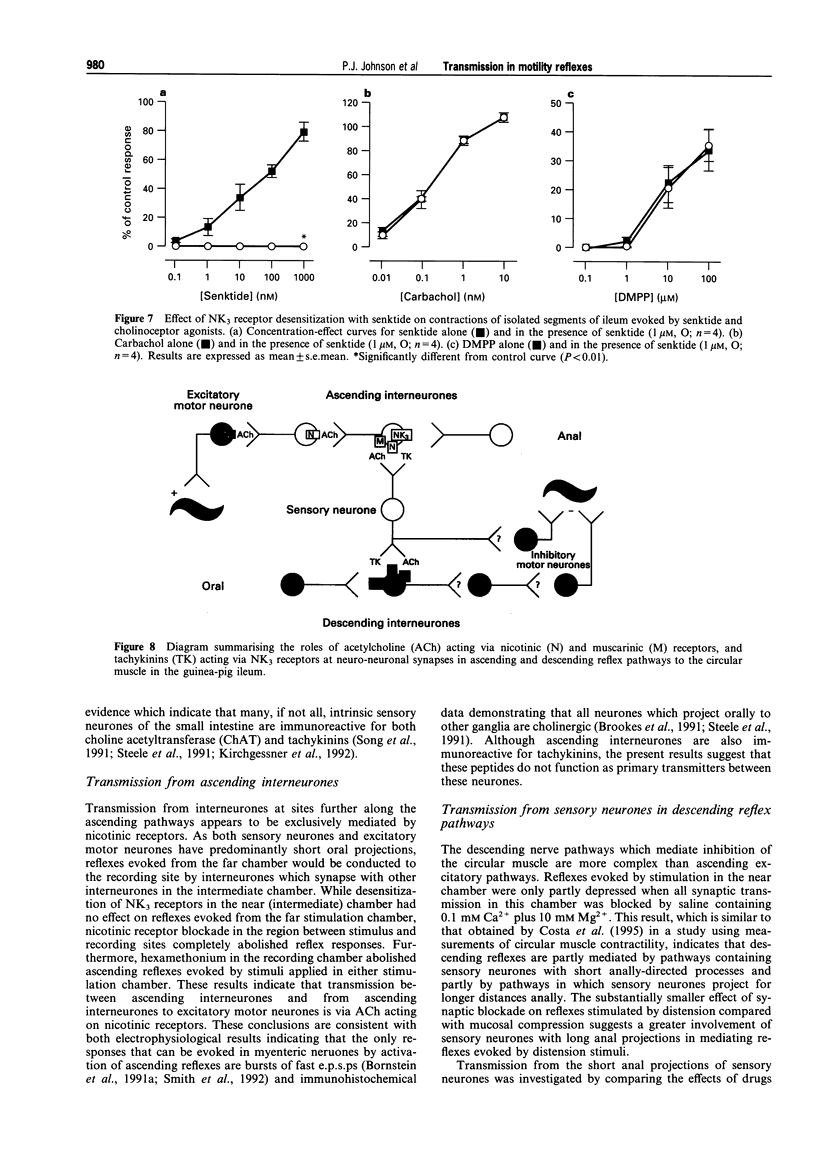



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. J Physiol. 1899 May 11;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Costa M., Furness J. B., Lang R. J. Electrophysiological analysis of projections of enteric inhibitory motoneurones in the guinea-pig small intestine. J Physiol. 1986 Jan;370:61–74. doi: 10.1113/jphysiol.1986.sp015922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Furness J. B., Smith T. K., Trussell D. C. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J Neurosci. 1991 Feb;11(2):505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Hendriks R., Furness J. B., Trussell D. C. Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J Comp Neurol. 1991 Dec 15;314(3):437–451. doi: 10.1002/cne.903140303. [DOI] [PubMed] [Google Scholar]
- Bornstein J. C. Local neural control of intestinal motility: nerve circuits deduced for the guinea-pig small intestine. Clin Exp Pharmacol Physiol. 1994 Jun;21(6):441–452. doi: 10.1111/j.1440-1681.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Brookes S. J., Steele P. A., Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42(3):863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Humphreys C. M. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976 Jul;294(1):47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Johnson P. J., Pompolo S., Bornstein J. C. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995 Jun;7(2):89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Bertrand P. P. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994 Dec;14(12):7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider J. R., Jin J. G. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994 May;14(5 Pt 1):2854–2860. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard S., Watson S. P. Evidence for neurokinin-3 receptor-mediated tachykinin release in the guinea-pig ileum. Eur J Pharmacol. 1987 Dec 15;144(3):409–412. doi: 10.1016/0014-2999(87)90398-0. [DOI] [PubMed] [Google Scholar]
- Guard S., Watson S. P., Maggio J. E., Too H. P., Watling K. J. Pharmacological analysis of [3H]-senktide binding to NK3 tachykinin receptors in guinea-pig ileum longitudinal muscle-myenteric plexus and cerebral cortex membranes. Br J Pharmacol. 1990 Apr;99(4):767–773. doi: 10.1111/j.1476-5381.1990.tb13004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKUHARA T., YAMAGAMI M., NAKAYAMA S. On the intestinal intrinsic reflexes. Jpn J Physiol. 1958 Mar 30;8(1):9–20. doi: 10.2170/jjphysiol.8.9. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., McKirdy H. C. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975 Jan;244(1):113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., Morita K., North R. A. Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. J Physiol. 1981 Nov;320:175–186. doi: 10.1113/jphysiol.1981.sp013942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Kirchgessner A. L., Tamir H., Gershon M. D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992 Jan;12(1):235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze W. A., Furness J. B., Bornstein J. C. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993 Aug;55(3):685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- Laufer R., Wormser U., Friedman Z. Y., Gilon C., Chorev M., Selinger Z. Neurokinin B is a preferred agonist for a neuronal substance P receptor and its action is antagonized by enkephalin. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7444–7448. doi: 10.1073/pnas.82.21.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Meini S., Giuliani S. Nitric oxide is the mediator of tachykinin NK3 receptor-induced relaxation in the circular muscle of the guinea-pig ileum. Eur J Pharmacol. 1993 Aug 10;240(1):45–50. doi: 10.1016/0014-2999(93)90543-q. [DOI] [PubMed] [Google Scholar]
- McConalogue K., Lyster D. J., Furness J. B. Electrophysiological analysis of the actions of pituitary adenylyl cyclase activating peptide in the taenia of the guinea-pig caecum. Naunyn Schmiedebergs Arch Pharmacol. 1995 Nov;352(5):538–544. doi: 10.1007/BF00169388. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Katayama Y. Evidence that substance P is a neurotransmitter in the myenteric plexus. Nature. 1980 Sep 11;287(5778):151–152. doi: 10.1038/287151a0. [DOI] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Muscarinic synaptic potentials in guinea-pig myenteric plexus neurones. J Physiol. 1982 Dec;333:151–156. doi: 10.1113/jphysiol.1982.sp014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Schemann M., Tamura K., Wood J. D. Calcitonin gene-related peptide excites myenteric neurons. Eur J Pharmacol. 1986 Dec 16;132(2-3):163–170. doi: 10.1016/0014-2999(86)90601-1. [DOI] [PubMed] [Google Scholar]
- Pompolo S., Furness J. B. Sources of inputs to longitudinal muscle motor neurons and ascending interneurons in the guinea-pig small intestine. Cell Tissue Res. 1995 Jun;280(3):549–560. doi: 10.1007/BF00318359. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Bornstein J. C., Furness J. B. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci. 1992 Apr;12(4):1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. K., Bornstein J. C., Furness J. B. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J Auton Nerv Syst. 1990 Mar;29(3):203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Bornstein J. C., Furness J. B. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991 Jun 1;34(1):69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Furness J. B. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J Auton Nerv Syst. 1988 Dec;25(2-3):205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Song Z. M., Brookes S. J., Costa M. Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neurosci Lett. 1991 Aug 19;129(2):294–298. doi: 10.1016/0304-3940(91)90484-b. [DOI] [PubMed] [Google Scholar]
- Steele P. A., Brookes S. J., Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45(1):227–239. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- Thore C. R., Nam M. J., Busija D. W. Immunofluorescent localization of constitutive and inducible prostaglandin H synthase in ovine astroglia. J Comp Neurol. 1996 Mar 25;367(1):1–9. doi: 10.1002/(SICI)1096-9861(19960325)367:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tonini M., Costa M. A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience. 1990;38(3):787–795. doi: 10.1016/0306-4522(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Tonini M., Frigo G., Lecchini S., D'Angelo L., Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. Eur J Pharmacol. 1981 May 22;71(4):375–381. doi: 10.1016/0014-2999(81)90181-3. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Slow synaptic excitation mediated by serotonin in Auerbach's plexus. Nature. 1978 Dec 21;276(5690):836–837. doi: 10.1038/276836a0. [DOI] [PubMed] [Google Scholar]
- Yagasaki O., Suzuki H., Yanagiya I. [Oral propagation of the circular muscle contraction induced by local distension of the isolated guinea pig ileum (author's transl)]. Nihon Heikatsukin Gakkai Zasshi. 1979 Dec;15(4):353–364. doi: 10.1540/jsmr1965.15.353. [DOI] [PubMed] [Google Scholar]
- Yau W. M., Mandel K. G., Dorsett J. A., Youther M. L. Neurokinin3 receptor regulation of acetylcholine release from myenteric plexus. Am J Physiol. 1992 Nov;263(5 Pt 1):G659–G664. doi: 10.1152/ajpgi.1992.263.5.G659. [DOI] [PubMed] [Google Scholar]
- Young H. M., Furness J. B., Povey J. M. Analysis of connections between nitric oxide synthase neurons in the myenteric plexus of the guinea-pig small intestine. J Neurocytol. 1995 Apr;24(4):257–263. doi: 10.1007/BF01186538. [DOI] [PubMed] [Google Scholar]
- Yuan S. Y., Bornstein J. C., Furness J. B. Investigation of the role of 5-HT3 and 5-HT4 receptors in ascending and descending reflexes to the circular muscle of guinea-pig small intestine. Br J Pharmacol. 1994 Aug;112(4):1095–1100. doi: 10.1111/j.1476-5381.1994.tb13196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. Y., Bornstein J. C., Furness J. B. Pharmacological evidence that nitric oxide may be a retrograde messenger in the enteric nervous system. Br J Pharmacol. 1995 Jan;114(2):428–432. doi: 10.1111/j.1476-5381.1995.tb13244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. Y., Furness J. B., Bornstein J. C., Smith T. K. Mucosal distortion by compression elicits polarized reflexes and enhances responses of the circular muscle to distension in the small intestine. J Auton Nerv Syst. 1991 Sep;35(3):219–226. doi: 10.1016/0165-1838(91)90100-h. [DOI] [PubMed] [Google Scholar]