Abstract
1. We and others have shown that extracellular ATP (adenosine triphosphate), released from sympathetic nerves and platelets, stimulates growth of vascular smooth muscle cells (SMC). To study the importance of tyrosine kinases for ATP-mediated proliferation in vascular smooth muscle cells we used tyrphostins, a recently developed group of highly specific inhibitors of tyrosine kinases. 2. ATP induced a powerful concentration-dependent increase in DNA synthesis measured by [3H]-thymidine incorporation in rat aorta SMC (RASMC) and an increase in total cell number after 72 h of incubation as measured by an enzymatic cell proliferation assay. Tyrphostin 25 (10(-5) M) had no effect per se on basal DNA synthesis but reduced ATP-stimulated DNA synthesis and increase in cell number in a dose-dependent manner. Higher concentrations of ATP could not reverse the inhibitory effect of tyrphostin 25. The potency of several (six) other tyrphostins was also examined and found to be slightly greater than tyrphostin 25 with equal efficacy. 3. When RASMC were incubated with 10(-5) M ATP for 2 h, nearly all of the cells (87 +/- 5%) were intensely stained with an antibody to the Fos protein while in the controls only 1 +/- 2% of the cells were weakly stained. Tyrphostin 25 greatly reduced the Fos-protein staining (14 +/- 2%). 4. ATP induced a concentration-dependent increase in 45Ca(2+)-influx and formation of inositol phosphates (IPtotal) in RASMC. These effects were not inhibited by tyrphostin 25. 5. Tyrphostin 25 did not alter ATP-induced contraction in ring segments of rat aorta. 6. In conclusion, tyrphostin 25 inhibited ATP-induced DNA synthesis, cell proliferation and Fos-protein expression, but not ATP-induced 45Ca(2+)-influx, inositolphosphate-production or vasoconstriction. This indicates that the mitogenic effect of ATP on vascular smooth muscle cells is dependent on tyrosine kinases in contrast to the contractile effect of ATP in blood vessels.
Full text
PDF



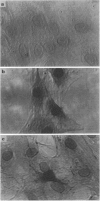
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anafi M., Gazit A., Gilon C., Ben-Neriah Y., Levitzki A. Selective interactions of transforming and normal abl proteins with ATP, tyrosine-copolymer substrates, and tyrphostins. J Biol Chem. 1992 Mar 5;267(7):4518–4523. [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder G. E., Krawiec J. A., McVety K., Gazit A., Gilon C., Lyall R., Zilberstein A., Levitzki A., Perrone M. H., Schreiber A. B. Tyrphostins inhibit PDGF-induced DNA synthesis and associated early events in smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C721–C730. doi: 10.1152/ajpcell.1991.260.4.C721. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Di Salvo J., Steusloff A., Semenchuk L., Satoh S., Kolquist K., Pfitzer G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun. 1993 Feb 15;190(3):968–974. doi: 10.1006/bbrc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Erlinge D., Brunkwall J., Edvinsson L. Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept. 1994 Mar 17;50(3):259–265. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Erlinge D., Yoo H., Edvinsson L., Reis D. J., Wahlestedt C. Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am J Physiol. 1993 Oct;265(4 Pt 2):H1089–H1097. doi: 10.1152/ajpheart.1993.265.4.H1089. [DOI] [PubMed] [Google Scholar]
- Erlinge D., You J., Wahlestedt C., Edvinsson L. Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol. 1995 Mar 15;289(1):135–149. doi: 10.1016/0922-4106(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Gazit A., Osherov N., Posner I., Yaish P., Poradosu E., Gilon C., Levitzki A. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem. 1991 Jun;34(6):1896–1907. doi: 10.1021/jm00110a022. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Bonni A., Greenberg M. E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994 Jun 3;77(5):713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Kornhauser J. M., Thompson M. A., Bading H., Mayo K. E., Takahashi J. S., Greenberg M. E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993 Apr 9;260(5105):238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D. Tyrosine kinase pathways and the regulation of smooth muscle contractility. Trends Pharmacol Sci. 1994 Apr;15(4):108–114. doi: 10.1016/0165-6147(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Huwiler A., Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol. 1994 Dec;113(4):1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Song X. H. Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem. 1991 Apr 25;266(12):7746–7749. [PubMed] [Google Scholar]
- Levitzki A., Gilon C. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol Sci. 1991 May;12(5):171–174. doi: 10.1016/0165-6147(91)90538-4. [DOI] [PubMed] [Google Scholar]
- Malam-Souley R., Campan M., Gadeau A. P., Desgranges C. Exogenous ATP induces a limited cell cycle progression of arterial smooth muscle cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C783–C788. doi: 10.1152/ajpcell.1993.264.4.C783. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Zhu Q. Signaling by ATP receptors in astrocytes. Neuroreport. 1994 Aug 15;5(13):1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H., Späth M., Schlunck G., Nauck M., Fischer R., Wanner C., Schollmeyer P. Effect of nucleotides on the cytosolic free calcium activity and inositol phosphate formation in human glomerular epithelial cells. Br J Pharmacol. 1992 Sep;107(1):189–195. doi: 10.1111/j.1476-5381.1992.tb14485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone M. P., Deforge S., Deluca B., Gabel B., Laurenssen C., Middlemiss P., Parkinson S. Purinergic stimulation of cell division and differentiation: mechanisms and pharmacological implications. Med Hypotheses. 1992 Apr;37(4):213–219. doi: 10.1016/0306-9877(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Miranti C. K., Misra R. P., Ginty D. D., Chen R. H., Blenis J., Greenberg M. E. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993 Oct;13(10):6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Regulation of c-myc and c-fos proto-oncogene expression by animal cell growth factors. In Vitro Cell Dev Biol. 1988 Feb;24(2):81–84. doi: 10.1007/BF02623883. [DOI] [PubMed] [Google Scholar]
- Ross R. Platelet-derived growth factor. Lancet. 1989 May 27;1(8648):1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- Sauro M. D., Thomas B. Tyrphostin attenuates platelet-derived growth factor-induced contraction in aortic smooth muscle through inhibition of protein tyrosine kinase(s). J Pharmacol Exp Ther. 1993 Dec;267(3):1119–1125. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Seckl M., Rozengurt E. Tyrphostin inhibits bombesin stimulation of tyrosine phosphorylation, c-fos expression, and DNA synthesis in Swiss 3T3 cells. J Biol Chem. 1993 May 5;268(13):9548–9554. [PubMed] [Google Scholar]
- Stjärne L., Lishajko F. Comparison of spontaneous loss of catecholamines and ATP in vitro from isolated bovine adrenomedullary, vesicular gland, vas deferens and splenic nerve granules. J Neurochem. 1966 Nov;13(11):1213–1216. doi: 10.1111/j.1471-4159.1966.tb04279.x. [DOI] [PubMed] [Google Scholar]
- Wang D. J., Huang N. N., Heppel L. A. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol. 1992 Nov;153(2):221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Stimulation of tyrosine kinase activity in anti-phosphotyrosine immune complexes of Swiss 3T3 cell lysates occurs rapidly after addition of bombesin, vasopressin, and endothelin to intact cells. J Biol Chem. 1991 Dec 15;266(35):24126–24133. [PubMed] [Google Scholar]