Abstract
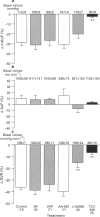
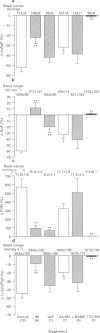
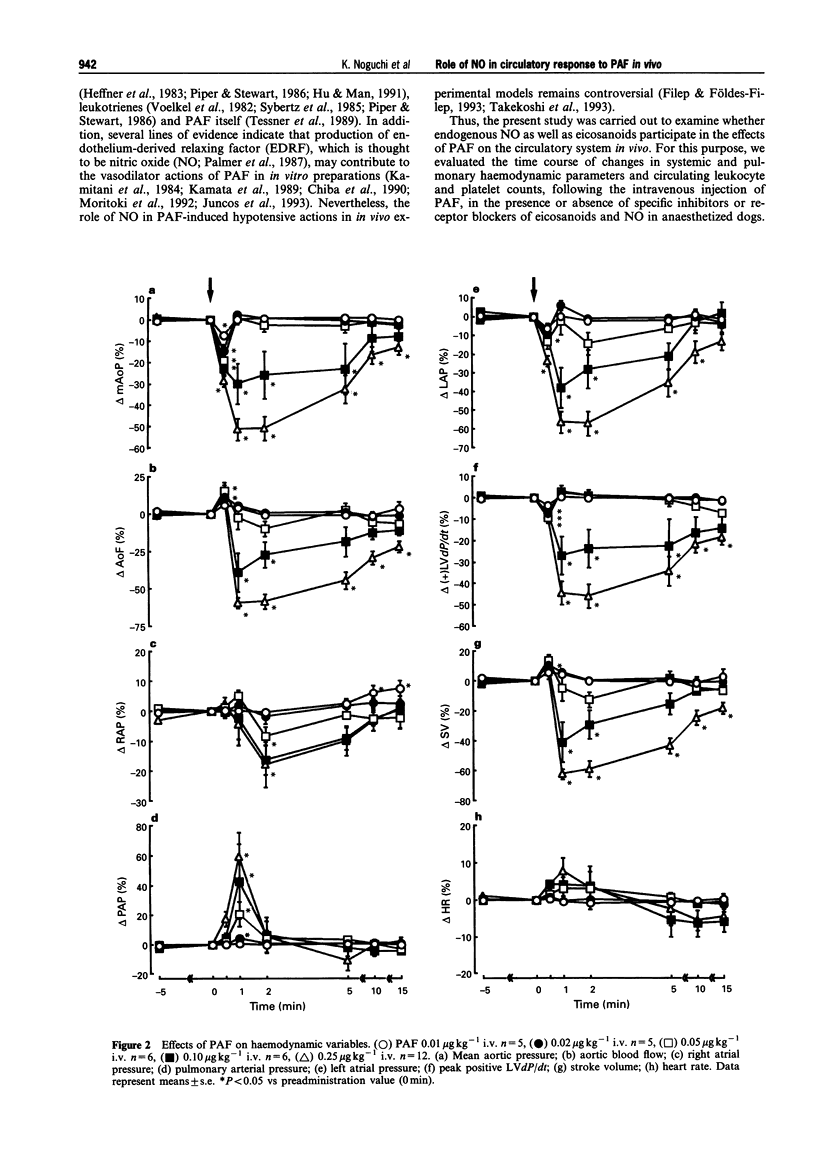
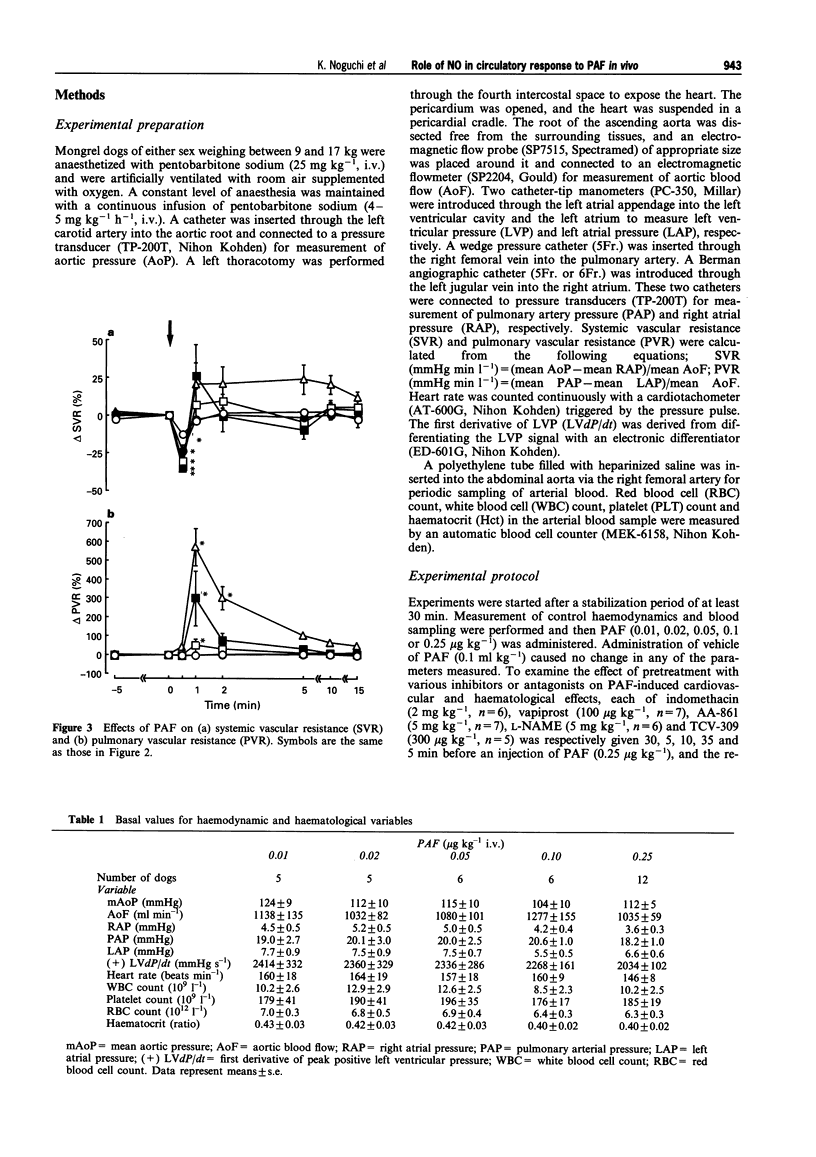
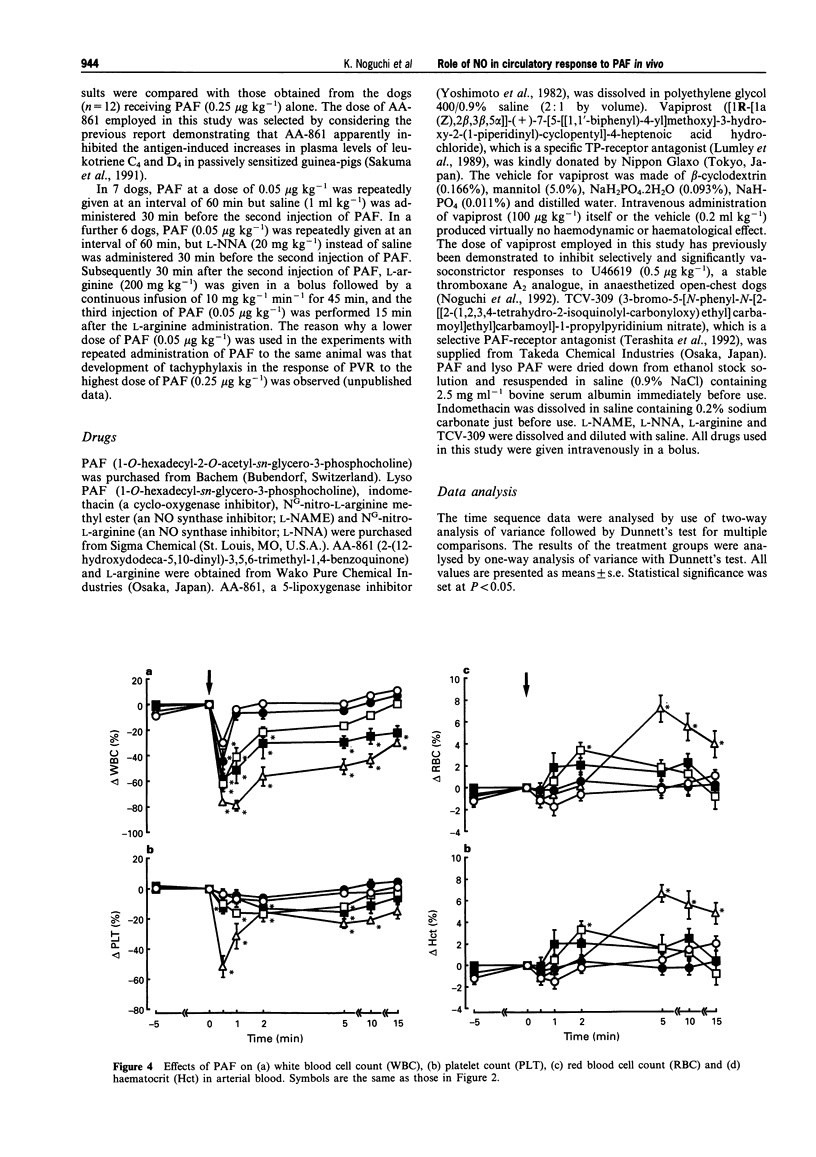
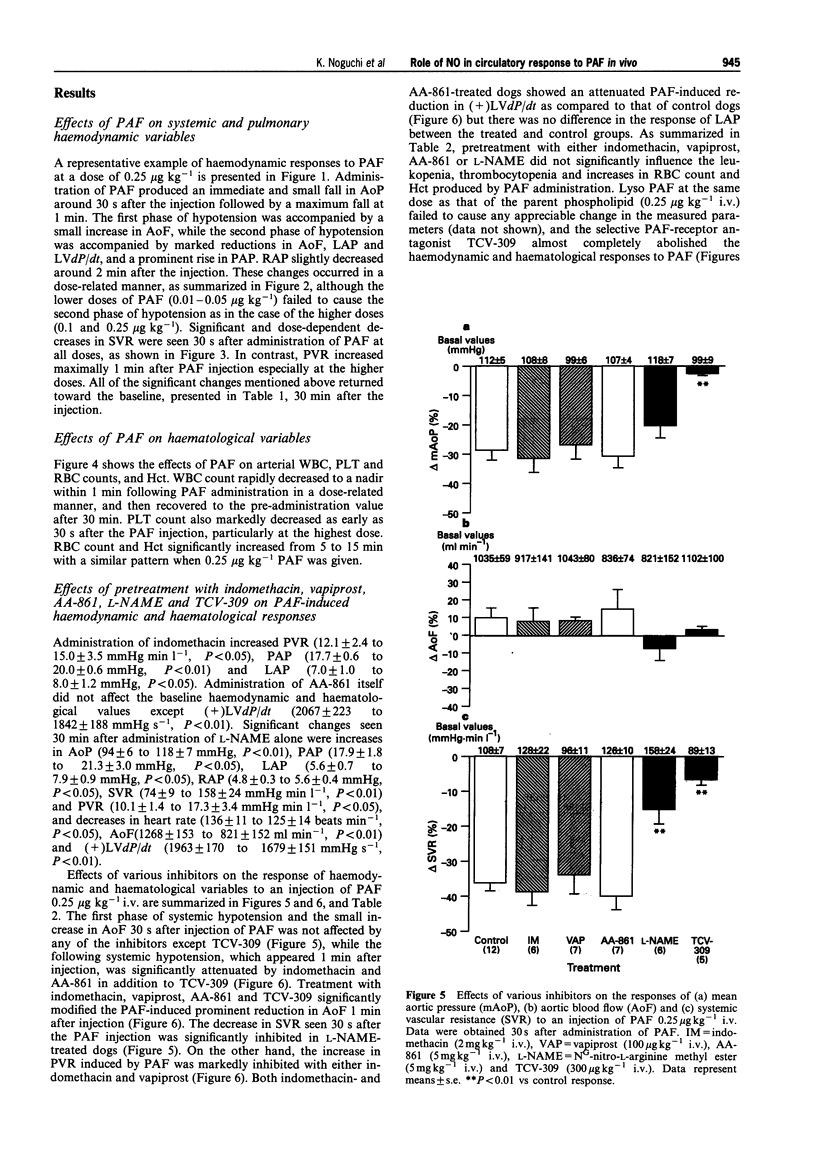
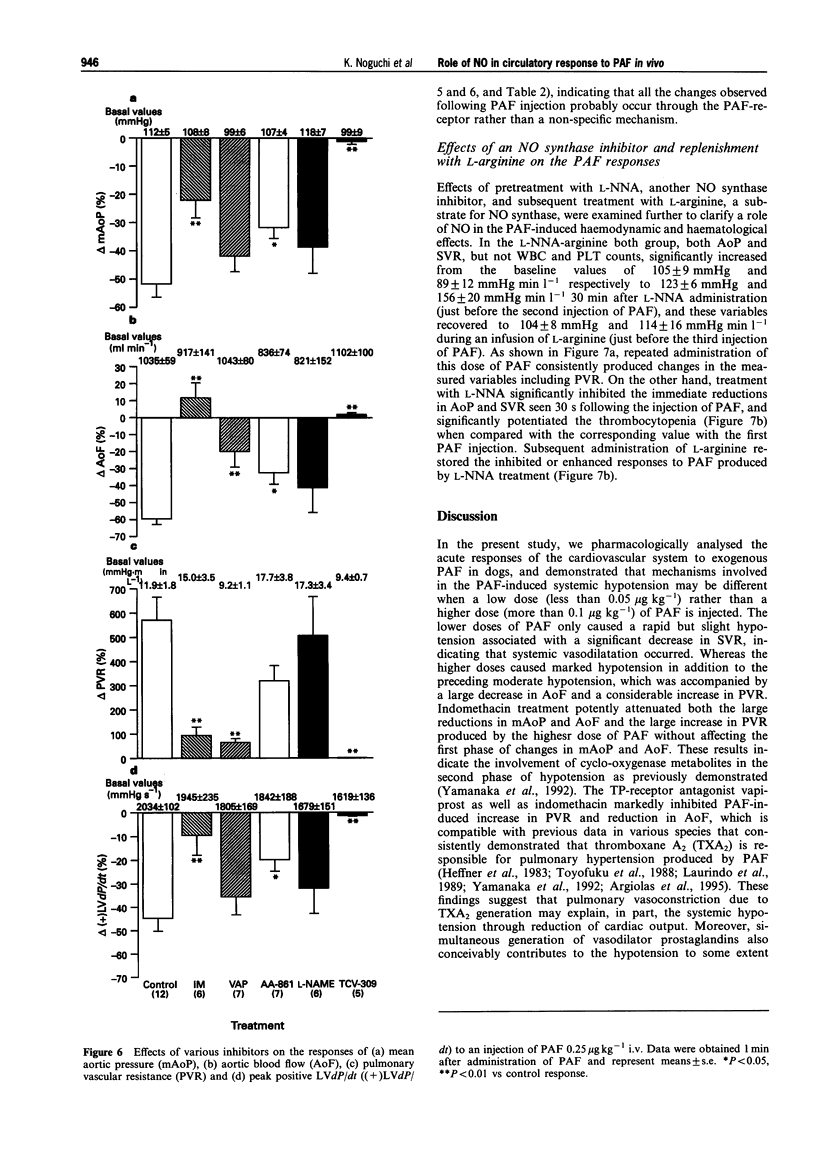
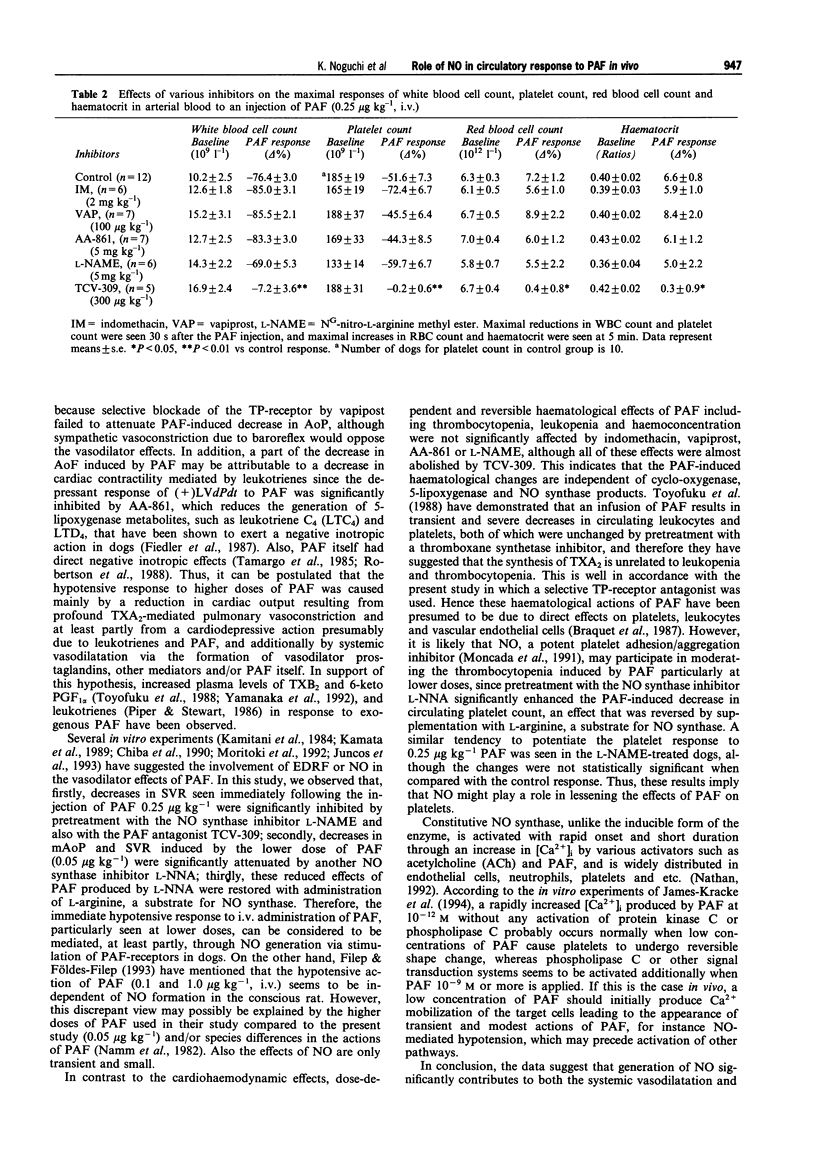
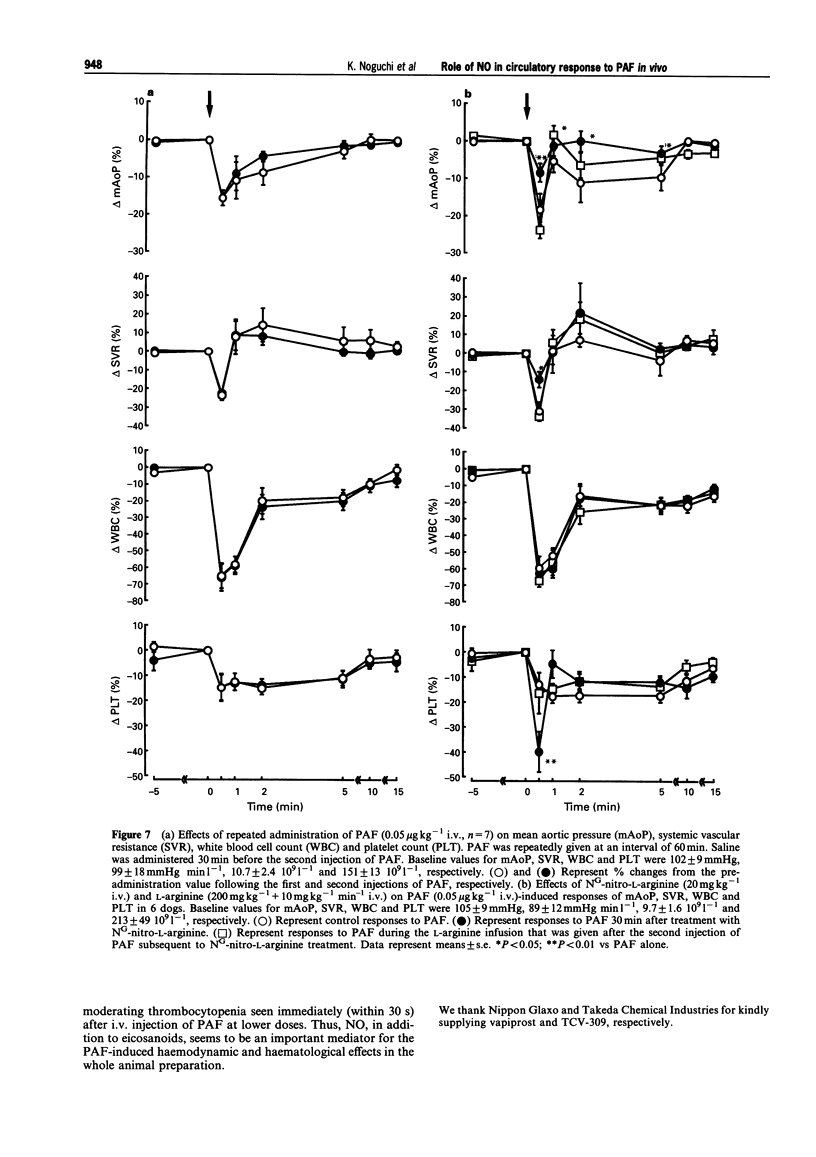
1. Platelet-activating factor (PAF) is a phospholipid mediator with potent cardiovascular and haematological actions. But its mechanisms of action in vivo have not been fully elucidated, probably due to difficulties arising from previous findings that the effects of PAF are largely mediated by the release of a variety of other autacoids. In the present study, the roles of nitric oxide and eicosanoids in the effects of PAF (0.01-0.25 microgram kg-1 i.v.) on systemic and pulmonary vasculatures and circulating blood cell count were pharmacologically evaluated in anaesthetized dogs. 2. Higher doses of PAF (> 0.1 microgram kg-1) produced a biphasic systemic hypotension. The first hypotension seen 30 s after the injection was accompanied by a decrease in systemic vascular resistance, thrombocytopenia and leukopenia, while the second hypotension seen 1-2 min after PAF was accompanied by a marked rise in pulmonary vascular resistance and decreases in aortic blood flow and cardiac contractility. Lower doses of PAF (0.01 - 0.05 microgram kg-1) caused only the first responses in a dose dependent manner. 3. Pretreatment with indomethacin inhibited the second responses to PAF without affecting the first responses. The thromboxane A2/prostaglandin H2 (TP)-receptor antagonist vapiprost blocked the PAF-induced rise in pulmonary vascular resistance. AA-861, a 5-lipoxygenase inhibitor, attenuated the PAF-induced cardiac depression. The nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester inhibited the PAF-induced early decrease in systemic vascular resistance. 4. All observed changes in haemodynamics and blood cell count after PAF were almost abolished by TCV-309, a PAF-receptor antagonist. 5. Reproducible hypotension and thrombocytopenia produced by a lower dose of PAF (0.05 microgram kg-1) were respectively attenuated and potentiated by pretreatment with NG-nitro-L-arginine, another nitric oxide synthase inhibitor. Administration of L-arginine reversed the effects of the nitric oxide synthase inhibitor. 6. These results indicate that PAF-receptor-mediated production of not only eicosanoids but also nitric oxide may contribute to the cardiovascular and haematological responses to PAF in the dog.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argiolas L., Fabi F., del Basso P. Mechanisms of pulmonary vasoconstriction and bronchoconstriction produced by PAF in the guinea-pig: role of platelets and cyclo-oxygenase metabolites. Br J Pharmacol. 1995 Jan;114(1):203–209. doi: 10.1111/j.1476-5381.1995.tb14926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessin P., Bonnet J., Apffel D., Soulard C., Desgroux L., Pelas I., Benveniste J. Acute circulatory collapse caused by platelet-activating factor (PAF-acether) in dogs. Eur J Pharmacol. 1983 Jan 21;86(3-4):403–413. doi: 10.1016/0014-2999(83)90190-5. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Chiba Y., Mikoda N., Kawasaki H., Ito K. Endothelium-dependent relaxant action of platelet activating factor in the rat mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):68–73. doi: 10.1007/BF00195060. [DOI] [PubMed] [Google Scholar]
- Fiedler V. B., Mardin M., Abram T. S. Comparison of cardiac and hemodynamic effects of platelet-activating factor-acether and leukotriene D4 in anesthetized dogs. Basic Res Cardiol. 1987 Mar-Apr;82(2):197–208. doi: 10.1007/BF01907067. [DOI] [PubMed] [Google Scholar]
- Filep J. G., Földes-Filep E. Modulation by nitric oxide of platelet-activating factor-induced albumin extravasation in the conscious rat. Br J Pharmacol. 1993 Dec;110(4):1347–1352. doi: 10.1111/j.1476-5381.1993.tb13967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley D. A., Arbeeny C. M., Lee M. L., Van Valen R. G., Saunders R. N. Effect of platelet activating factor on endothelial permeability to plasma macromolecules. Immunopharmacology. 1984 Dec;8(3-4):137–142. doi: 10.1016/0162-3109(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. M., Man R. Y. Interaction of vasoactive substances released by platelet-activating factor in the rat perfused heart. Br J Pharmacol. 1991 Dec;104(4):933–937. doi: 10.1111/j.1476-5381.1991.tb12529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Kracke M. R., Sexe R. B., Shukla S. D. Picomolar platelet-activating factor mobilizes Ca to change platelet shape without activating phospholipase C or protein kinase C; simultaneous fluorometric measurement of intracellular free Ca concentration and aggregation. J Pharmacol Exp Ther. 1994 Nov;271(2):824–831. [PubMed] [Google Scholar]
- Juncos L. A., Ren Y. L., Arima S., Ito S. Vasodilator and constrictor actions of platelet-activating factor in the isolated microperfused afferent arteriole of the rabbit kidney. Role of endothelium-derived relaxing factor/nitric oxide and cyclooxygenase products. J Clin Invest. 1993 Apr;91(4):1374–1379. doi: 10.1172/JCI116339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Mori T., Shigenobu K., Kasuya Y. Endothelium-dependent vasodilator effects of platelet activating factor on rat resistance vessels. Br J Pharmacol. 1989 Dec;98(4):1360–1364. doi: 10.1111/j.1476-5381.1989.tb12685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani T., Katamoto M., Tatsumi M., Katsuta K., Ono T., Kikuchi H., Kumada S. Mechanism(s) of the hypotensive effect of synthetic 1-O-octadecyl-2-O-acetyl-glycero-3-phosphorylcholine. Eur J Pharmacol. 1984 Mar 2;98(3-4):357–366. doi: 10.1016/0014-2999(84)90284-x. [DOI] [PubMed] [Google Scholar]
- Kenzora J. L., Pérez J. E., Bergmann S. R., Lange L. G. Effects of acetyl glyceryl ether of phosphorylcholine (platelet activating factor) on ventricular preload, afterload, and contractility in dogs. J Clin Invest. 1984 Oct;74(4):1193–1203. doi: 10.1172/JCI111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. M., Shepherd C. A., Cervoni P., Wissner A. Hypotensive and vasodilatory activity of (+/-) 1-o-octadecyl-2-acetyl glyceryl-3-phosphorylcholine in the normotensive rat. Life Sci. 1983 Mar 7;32(10):1159–1166. doi: 10.1016/0024-3205(83)90122-4. [DOI] [PubMed] [Google Scholar]
- Laurindo F. R., Goldstein R. E., Davenport N. J., Ezra D., Feuerstein G. Z. Mechanisms of hypotension produced by platelet-activating factor. J Appl Physiol (1985) 1989 Jun;66(6):2681–2690. doi: 10.1152/jappl.1989.66.6.2681. [DOI] [PubMed] [Google Scholar]
- Lumley P., White B. P., Humphrey P. P. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br J Pharmacol. 1989 Jul;97(3):783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Demopoulos C. A., Pinckard R. N. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J Immunol. 1980 Jun;124(6):2919–2924. [PubMed] [Google Scholar]
- McManus L. M., Pinckard R. N., Fitzpatrick F. A., O'Rourke R. A., Crawford M. H., Hanahan D. J. Acetyl glyceryl ether phosphorylcholine. Intravascular alterations following intravenous infusion into the baboon. Lab Invest. 1981 Oct;45(4):303–307. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moritoki H., Hisayama T., Takeuchi S., Miyano H., Kondoh W. Involvement of nitric oxide pathway in the PAF-induced relaxation of rat thoracic aorta. Br J Pharmacol. 1992 Sep;107(1):196–201. doi: 10.1111/j.1476-5381.1992.tb14486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namm D. H., Tadepalli A. S., High J. A. Species specificity of the platelet responses to 1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine. Thromb Res. 1982 Feb 15;25(4):341–350. doi: 10.1016/0049-3848(82)90234-1. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Noguchi K., Ojiri Y., Chibana T., Matsuzaki T., Sakanashi M. Regional vascular responses to thromboxane A2 analogue and their blockade with vapiprost, a selective thromboxane receptor blocking drug, in anesthetized dogs. Jpn J Pharmacol. 1992 Dec;60(4):341–348. doi: 10.1254/jjp.60.341. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Stewart A. G. Coronary vasoconstriction in the rat, isolated perfused heart induced by platelet-activating factor is mediated by leukotriene C4. Br J Pharmacol. 1986 Jul;88(3):595–605. doi: 10.1111/j.1476-5381.1986.tb10240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. A., Wang D. Y., Lee C. O., Levi R. Negative inotropic effect of platelet-activating factor: association with a decrease in intracellular sodium activity. J Pharmacol Exp Ther. 1988 Apr;245(1):124–128. [PubMed] [Google Scholar]
- Sakuma Y., Tsunoda H., Katayama S., Abe S., Yamatsu I., Katayama K. Determination of plasma leukotrienes in antigen-induced bronchoconstrictive guinea pigs. Prostaglandins. 1991 Apr;41(4):315–329. doi: 10.1016/0090-6980(91)90002-w. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992 Mar;6(6):2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- Sybertz E. J., Watkins R. W., Baum T., Pula K., Rivelli M. Cardiac, coronary and peripheral vascular effects of acetyl glyceryl ether phosphoryl choline in the anesthetized dog. J Pharmacol Exp Ther. 1985 Jan;232(1):156–162. [PubMed] [Google Scholar]
- Takekoshi K., Kasai K., Suzuki Y., Sekiguchi Y., Banba N., Nakamura T., Shimoda S. Effect of NG-nitro-L-arginine on shock induced by endotoxin and by platelet activating factor in dogs. Eur J Pharmacol. 1993 Dec 21;250(3):465–467. doi: 10.1016/0014-2999(93)90035-g. [DOI] [PubMed] [Google Scholar]
- Tamargo J., Tejerina T., Delgado C., Barrigon S. Electrophysiological effects of platelet-activating factor (PAF-acether) in guinea-pig papillary muscles. Eur J Pharmacol. 1985 Feb 26;109(2):219–227. doi: 10.1016/0014-2999(85)90423-6. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Kawamura M., Takatani M., Tsushima S., Imura Y., Nishikawa K. Beneficial effects of TCV-309, a novel potent and selective platelet activating factor antagonist in endotoxin and anaphylactic shock in rodents. J Pharmacol Exp Ther. 1992 Feb;260(2):748–755. [PubMed] [Google Scholar]
- Tessner T. G., O'Flaherty J. T., Wykle R. L. Stimulation of platelet-activating factor synthesis by a nonmetabolizable bioactive analog of platelet-activating factor and influence of arachidonic acid metabolites. J Biol Chem. 1989 Mar 25;264(9):4794–4799. [PubMed] [Google Scholar]
- Toyofuku T., Kobayashi T., Koyama S., Kusama S. Pulmonary vascular response to platelet-activating factor in conscious sheep. Am J Physiol. 1988 Sep;255(3 Pt 2):H434–H440. doi: 10.1152/ajpheart.1988.255.3.H434. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Worthen S., Reeves J. T., Henson P. M., Murphy R. C. Nonimmunological production of leukotrienes induced by platelet-activating factor. Science. 1982 Oct 15;218(4569):286–289. doi: 10.1126/science.7123233. [DOI] [PubMed] [Google Scholar]
- Yamanaka S., Miura K., Yukimura T., Okumura M., Yamamoto K. Putative mechanism of hypotensive action of platelet-activating factor in dogs. Circ Res. 1992 May;70(5):893–901. doi: 10.1161/01.res.70.5.893. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Yokoyama C., Ochi K., Yamamoto S., Maki Y., Ashida Y., Terao S., Shiraishi M. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim Biophys Acta. 1982 Nov 12;713(2):470–473. [PubMed] [Google Scholar]