Abstract
1. The regulation of histamine-induced [3H]-inositol phosphate and intracellular calcium responses in human cultured airway smooth muscle cells was studied. 2. Histamine induced concentration-dependent [3H]-inositol phosphate formation (EC50 4 microM). This response was inhibited by a range of selective H1 receptor antagonists but not by the H2-selective antagonist, tiotidone or the H3 receptor-selective antagonist, thioperamide, indicating that an H1 receptor is involved in this response in human cultured airway smooth muscle cells. 3. Preincubation of human cultured airway smooth muscle cells with concentrations of dexamethasone > 10 nM for 22 h produced concentration-dependent inhibition of histamine-induced inositol phosphate formation. The maximum inhibition observed was 45% of the response in control cells. The inhibitory effect of dexamethasone was itself reversed by prior exposure to the glucocorticoid receptor antagonist, RU38486 (10 microM). Preincubation for 22 h with 1 microM dexamethasone produced inhibition of the inositol phosphate response to histamine to all concentrations of histamine inducing significant inositol phosphate formation in these cells. In contrast, the response to the G protein activator, NaF (0.1-20 mM) was unaltered by preincubation with dexamethasone. 4. Preincubation of human airway smooth muscle cells with 1 microM dexamethasone for time periods of < 6 h failed to inhibit histamine-induced inositol phosphate formation in human airway smooth muscle cells. 5. Histamine also induced concentration-dependent elevation of intracellular calcium levels in Fura 2-loaded human airway smooth muscle cells. This response was inhibited by preincubation with 1 microM dexamethasone. 6. We conclude that signal transduction through the H1 receptor in human airway smooth muscle is subject to regulation by dexamethasone and that this may in part account for the protective effect of dexamethasone against spasmogen-induced contractile responses in the airways.
Full text
PDF

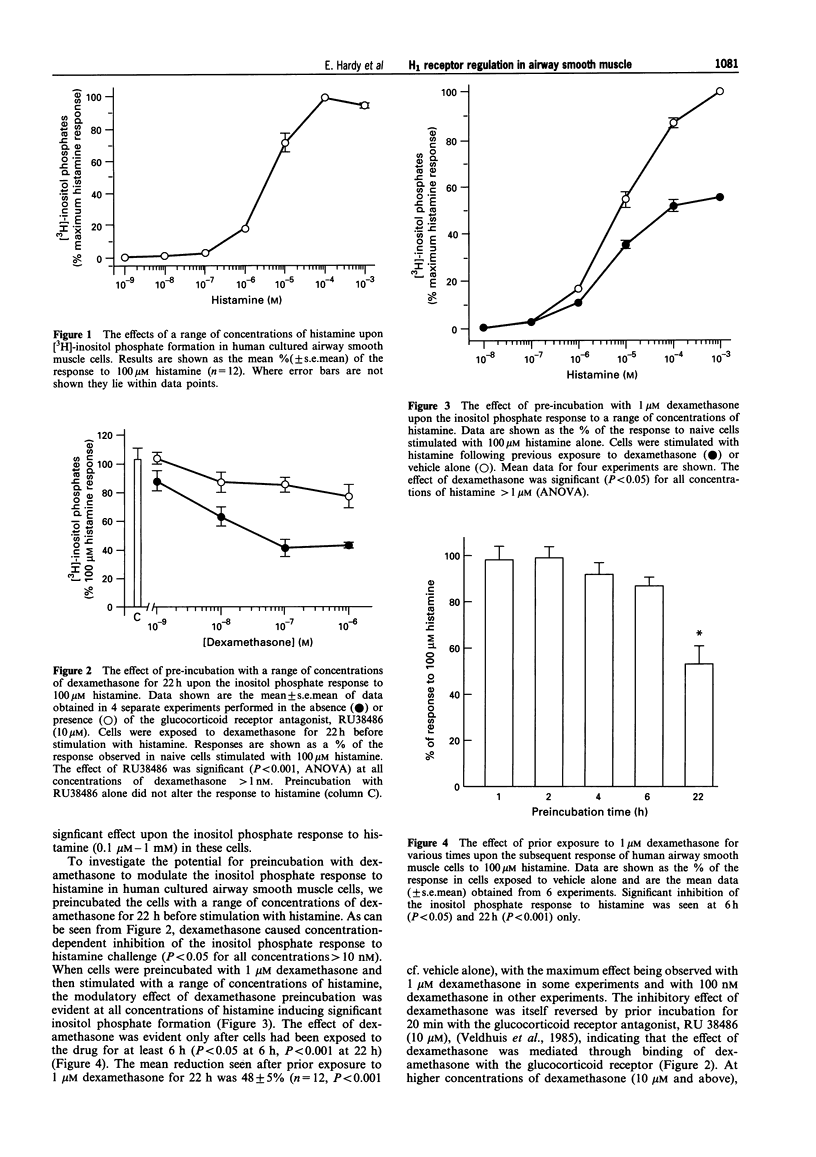
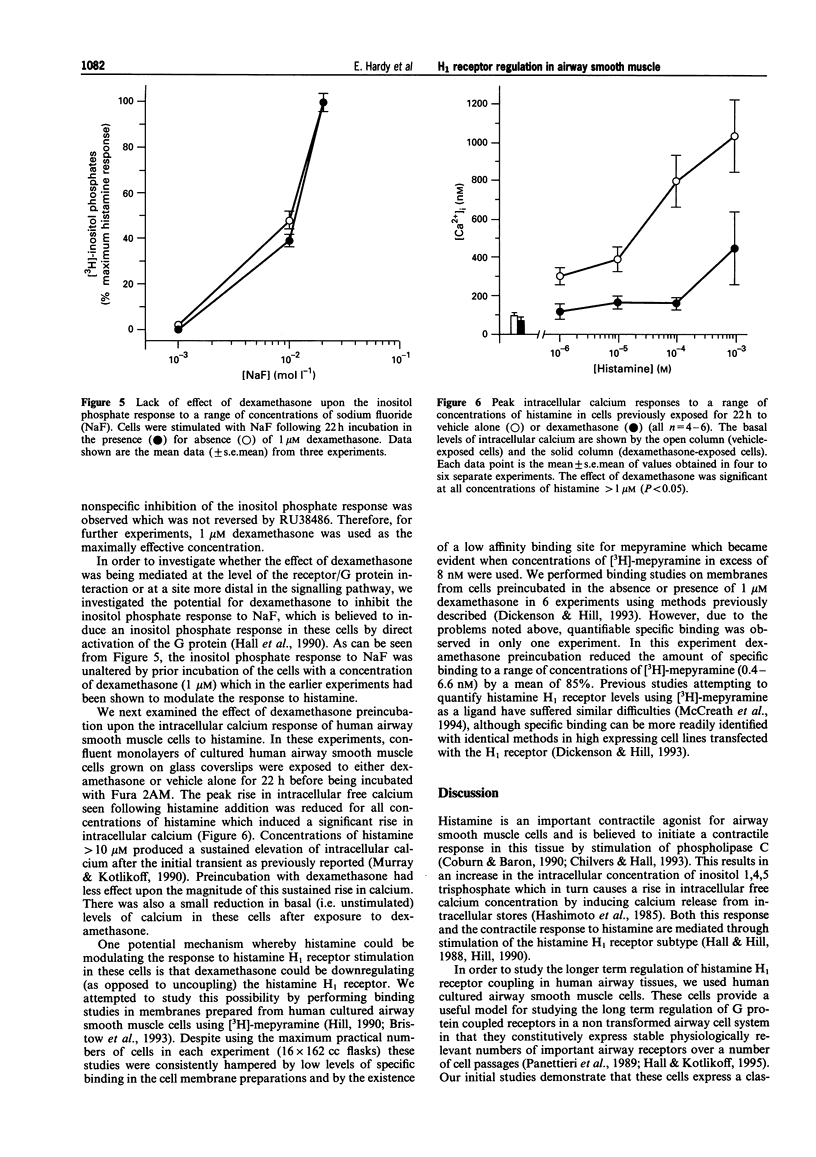


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bristow D. R., Banford P. C., Bajusz I., Vedat A., Young J. M. Desensitization of histamine H1 receptor-mediated inositol phosphate accumulation in guinea pig cerebral cortex slices. Br J Pharmacol. 1993 Sep;110(1):269–274. doi: 10.1111/j.1476-5381.1993.tb13804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daykin K., Widdop S., Hall I. P. Control of histamine induced inositol phospholipid hydrolysis in cultured human tracheal smooth muscle cells. Eur J Pharmacol. 1993 Jul 15;246(2):135–140. doi: 10.1016/0922-4106(93)90090-v. [DOI] [PubMed] [Google Scholar]
- De Backer M. D., Gommeren W., Moereels H., Nobels G., Van Gompel P., Leysen J. E., Luyten W. H. Genomic cloning, heterologous expression and pharmacological characterization of a human histamine H1 receptor. Biochem Biophys Res Commun. 1993 Dec 30;197(3):1601–1608. doi: 10.1006/bbrc.1993.2662. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Homologous and heterologous desensitization of histamine H1- and ATP-receptors in the smooth muscle cell line, DDT1MF-2: the role of protein kinase C. Br J Pharmacol. 1993 Dec;110(4):1449–1456. doi: 10.1111/j.1476-5381.1993.tb13984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzke E., Dieter P. Glucocorticoids inhibit formation of inositol phosphates in macrophages. Biochem Biophys Res Commun. 1991 Aug 15;178(3):974–979. doi: 10.1016/0006-291x(91)90987-i. [DOI] [PubMed] [Google Scholar]
- Fukui H., Fujimoto K., Mizuguchi H., Sakamoto K., Horio Y., Takai S., Yamada K., Ito S. Molecular cloning of the human histamine H1 receptor gene. Biochem Biophys Res Commun. 1994 Jun 15;201(2):894–901. doi: 10.1006/bbrc.1994.1786. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of fluoroaluminate-induced inositol phosphate formation by increases in tissue cyclic AMP content in bovine tracheal smooth muscle. Br J Pharmacol. 1990 Jul;100(3):646–650. doi: 10.1111/j.1476-5381.1990.tb15861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Hill S. J. Beta-adrenoceptor stimulation inhibits histamine-stimulated inositol phospholipid hydrolysis in bovine tracheal smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1204–1212. doi: 10.1111/j.1476-5381.1988.tb11757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Liu J., Haigh R. M., Jones C. T. Enhancement of noradrenaline-induced inositol polyphosphate formation by glucocorticoids in rat vascular smooth muscle cells. J Endocrinol. 1992 Jun;133(3):405–411. doi: 10.1677/joe.0.1330405. [DOI] [PubMed] [Google Scholar]
- McCreath G., Hall I. P., Hill S. J. Agonist-induced desensitization of histamine H1 receptor-mediated inositol phospholipid hydrolysis in human umbilical vein endothelial cells. Br J Pharmacol. 1994 Nov;113(3):823–830. doi: 10.1111/j.1476-5381.1994.tb17067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs H., Timmermans A., Van Amsterdam R. G., Brouwer F., Kauffman H. F., Zaagsma J. Muscarinic receptors in human airway smooth muscle are coupled to phosphoinositide metabolism. Eur J Pharmacol. 1989 May 19;164(2):369–371. doi: 10.1016/0014-2999(89)90480-9. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabishah B. M., Morat P. B., Khalid B. A., Kadir B. A. Effect of acetylcholine and morphine on bronchial smooth muscle contraction and its modulation by steroid hormones. Clin Exp Pharmacol Physiol. 1990 Dec;17(12):841–847. doi: 10.1111/j.1440-1681.1990.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Nambi P., Pullen M., Wu H. L., Nuthulaganti P., Elshourbagy N., Kumar C. Dexamethasone down-regulates the expression of endothelin receptors in vascular smooth muscle cells. J Biol Chem. 1992 Sep 25;267(27):19555–19559. [PubMed] [Google Scholar]
- Panettieri R. A., Murray R. K., DePalo L. R., Yadvish P. A., Kotlikoff M. I. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989 Feb;256(2 Pt 1):C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- Sato A., Suzuki H., Iwaita Y., Nakazato Y., Kato H., Saruta T. Dexamethasone potentiates production of inositol trisphosphate evoked by endothelin-1 in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1992 Aug;20(2):290–295. doi: 10.1097/00005344-199208000-00015. [DOI] [PubMed] [Google Scholar]
- Veldhuis H. D., De Korte C. C., De Kloet E. R. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol. 1985 Sep 24;115(2-3):211–217. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- Yasunari K., Kohno M., Yokokawa K., Horio T., Takeda T. Dopamine DA1 receptors on vascular smooth muscle cells are regulated by glucocorticoid and sodium chloride. Am J Physiol. 1994 Sep;267(3 Pt 2):R628–R634. doi: 10.1152/ajpregu.1994.267.3.R628. [DOI] [PubMed] [Google Scholar]


