Abstract
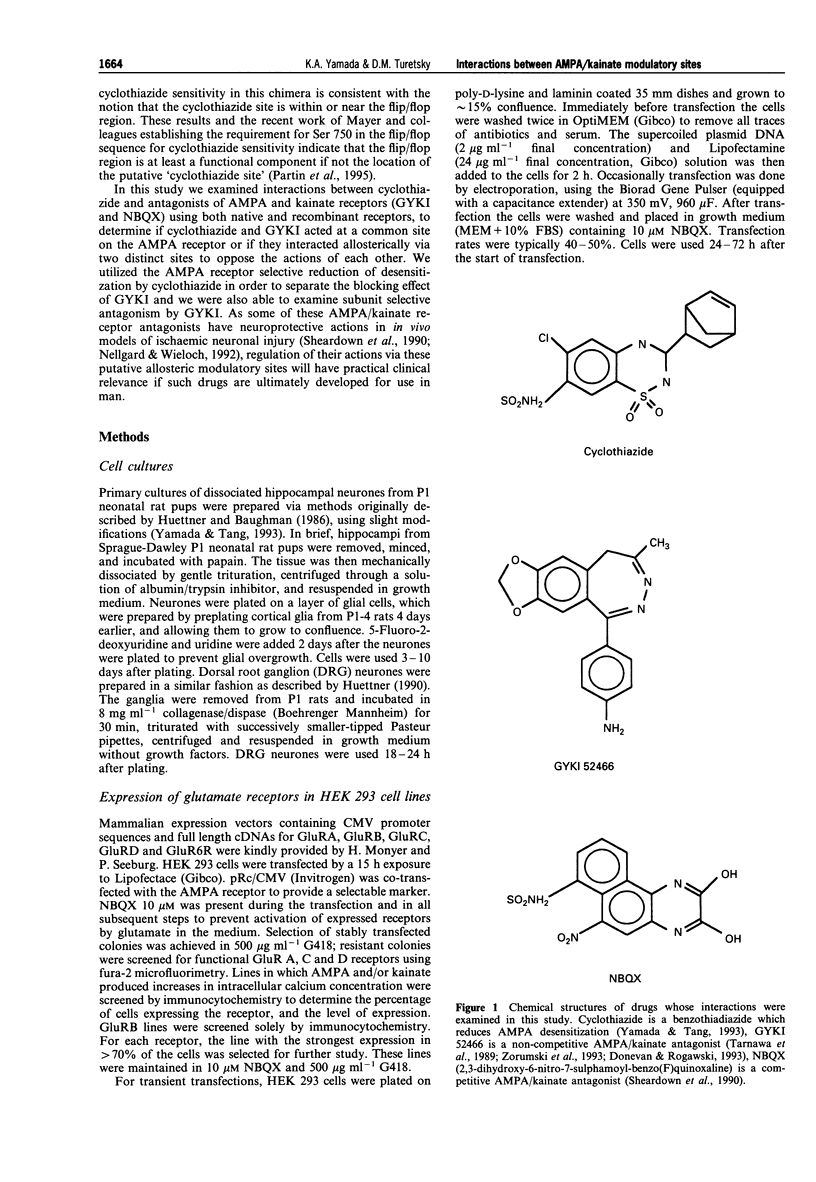
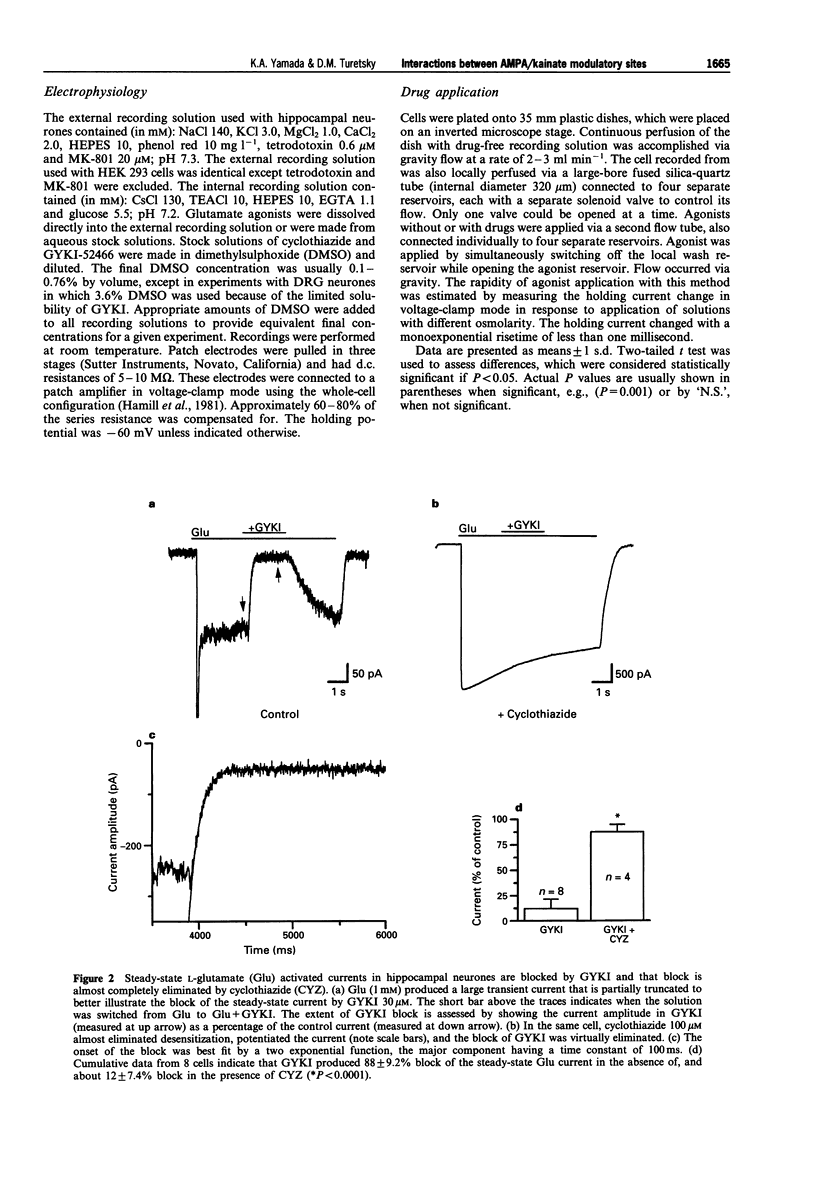
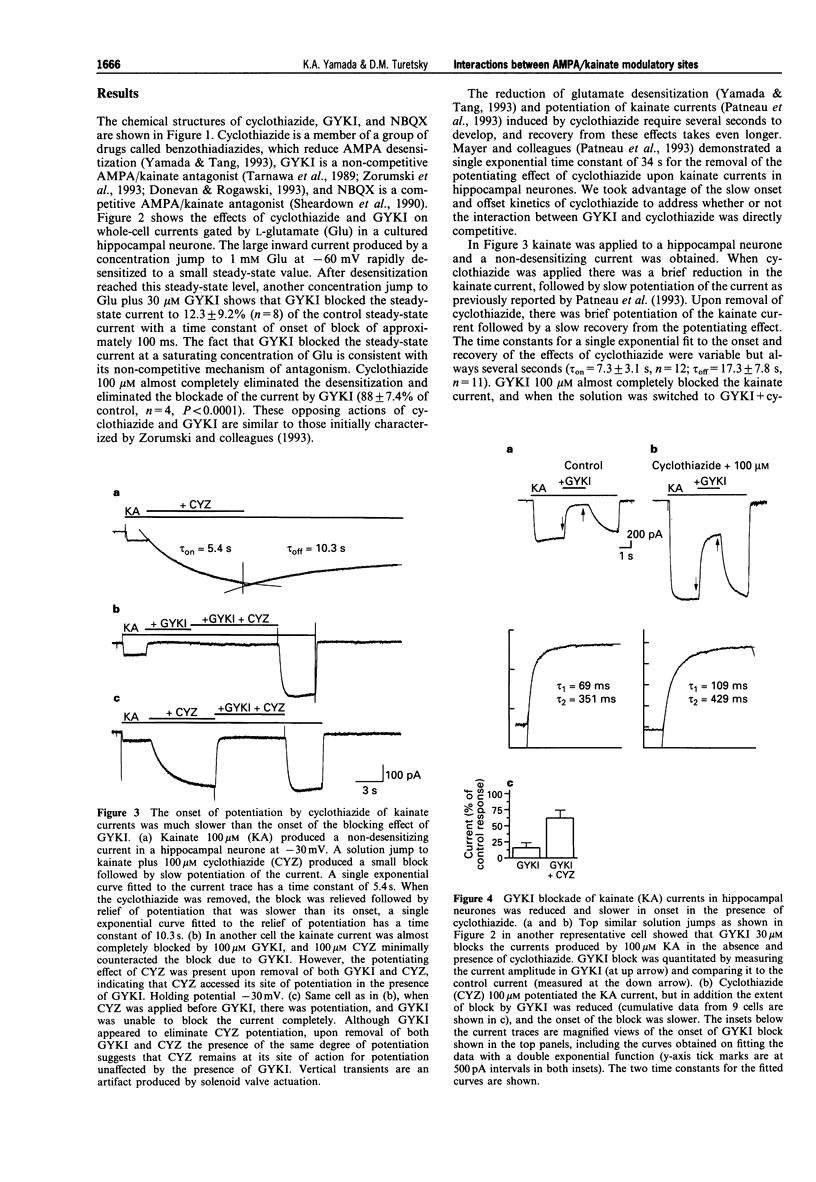
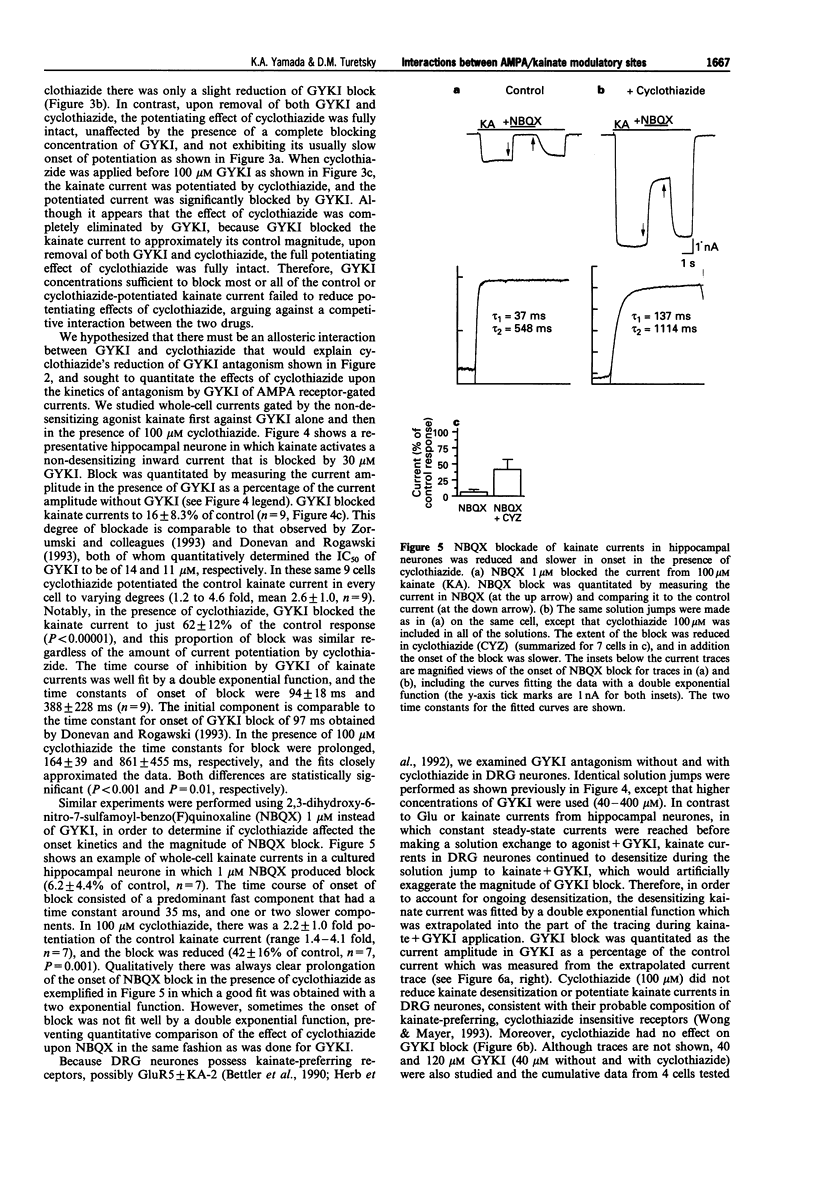
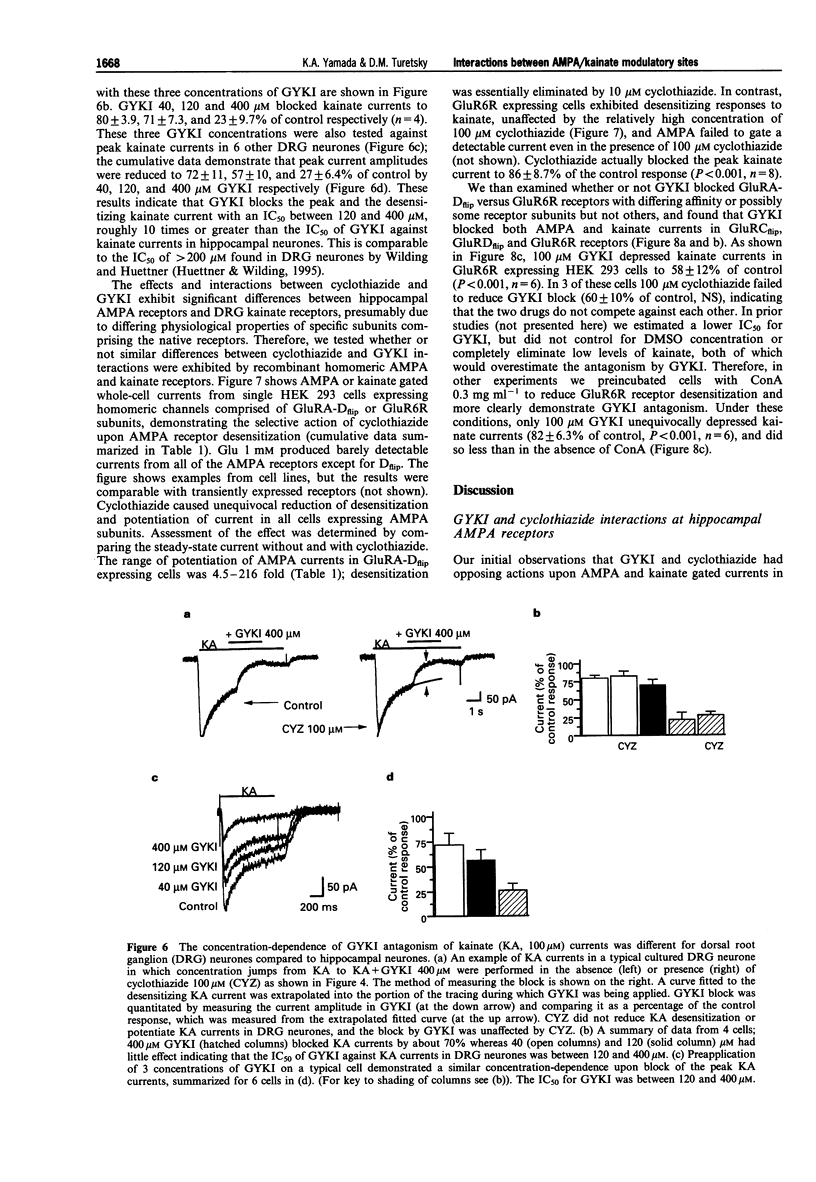
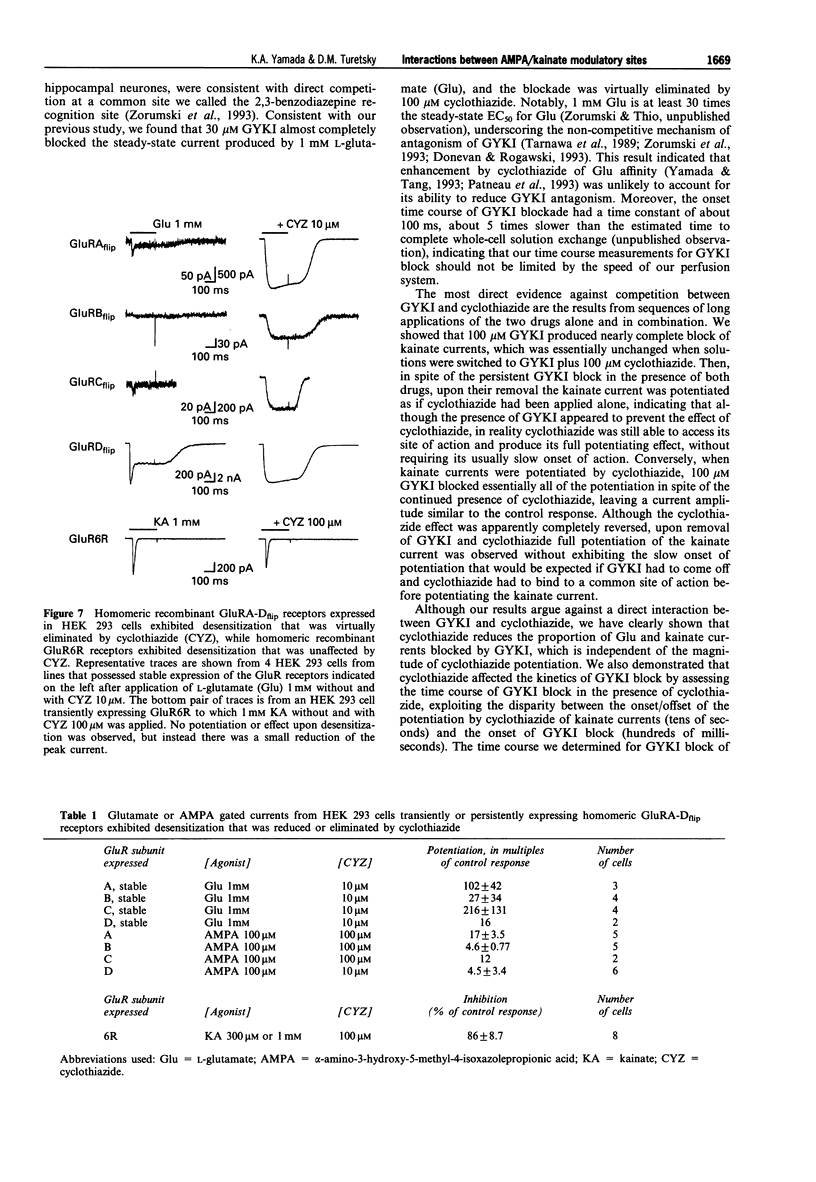
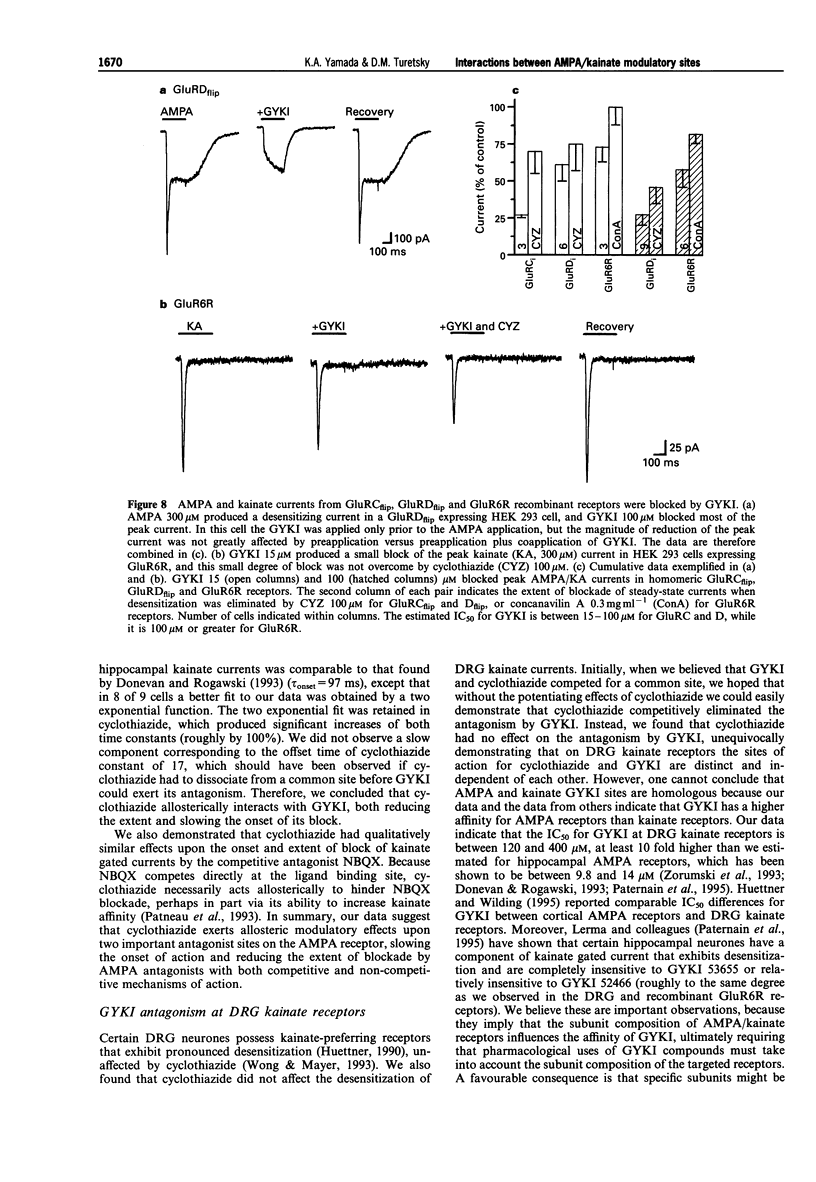
1. Cyclothiazide blocks alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization and potentiates AMPA receptor gated currents. Interactions between cyclothiazide, and the non-competitive antagonist GYKI52466 (GYKI) and competitive antagonist 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo (F) quinoxaline (NBQX) were studied at native and recombinant AMPA/kainate receptors using whole-cell recording in order to characterize the modulation by cyclothiazide of these two antagonist sites. 2. GYKI 100 microM, which is sufficient to eliminate virtually hippocampal kainate (100 microM) currents, failed to prevent access of cyclothiazide to its site of potentiation, and was unable to enhance removal of cyclothiazide potentiation. However, cyclothiazide reduced GYKI (30 microM) block from 84 +/- 8.3% to 38 +/- 12%, and slowed the onset of the block with a time course much faster than the time course for onset and offset of potentiation induced by cyclothiazide. Cyclothiazide had qualitatively similar effects upon antagonism by NBQX 1 microM. 3. Kainate activated desensitizing currents in dorsal root ganglion (DRG) neurones, which were unaffected by cyclothiazide. GYKI blocked these kainate currents with lower affinity (IC50 > 120 microM) than for hippocampal neurones (IC50 < 30 microM), and cyclothiazide did not affect GYKI antagonism. 4. Steady-state AMPA currents from homomeric GluRA-Dflip receptors in HEK 293 cells were dramatically potentiated (up to 216 fold) by cyclothiazide via reduction of desensitization. In contrast, kainate-gated currents in HEK 293 cells expressing GluR6R receptors exhibited pronounced desensitization that was unaffected by cyclothiazide. GYKI retains its inhibition at both recombinant AMPA and kainate receptors. 5. These results indicate that cyclothiazide allosterically influences two important antagonist sites on AMPA receptors. In addition, AMPA/kainate receptor subunit composition influences the affinity of GYKI for the receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herb A., Burnashev N., Werner P., Sakmann B., Wisden W., Seeburg P. H. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992 Apr;8(4):775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990 Sep;5(3):255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Johansen T. H., Chaudhary A., Verdoorn T. A. Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol Pharmacol. 1995 Nov;48(5):946–955. [PubMed] [Google Scholar]
- Jonas P., Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992 Sep;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kessler M., Arai A., Quan A., Lynch G. Effect of cyclothiazide on binding properties of AMPA-type glutamate receptors: lack of competition between cyclothiazide and GYKI 52466. Mol Pharmacol. 1996 Jan;49(1):123–131. [PubMed] [Google Scholar]
- Livsey C. T., Costa E., Vicini S. Glutamate-activated currents in outside-out patches from spiny versus aspiny hilar neurons of rat hippocampal slices. J Neurosci. 1993 Dec;13(12):5324–5333. doi: 10.1523/JNEUROSCI.13-12-05324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H., Mosbacher J., Melcher T., Höger T., Geiger J. R., Kuner T., Monyer H., Higuchi M., Bach A., Seeburg P. H. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994 Dec 9;266(5191):1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Moudy A. M., Yamada K. A., Rothman S. M. Rapid desensitization determines the pharmacology of glutamate neurotoxicity. Neuropharmacology. 1994 Aug;33(8):953–962. doi: 10.1016/0028-3908(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Nellgård B., Wieloch T. Postischemic blockade of AMPA but not NMDA receptors mitigates neuronal damage in the rat brain following transient severe cerebral ischemia. J Cereb Blood Flow Metab. 1992 Jan;12(1):2–11. doi: 10.1038/jcbfm.1992.2. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Bowie D., Mayer M. L. Structural determinants of allosteric regulation in alternatively spliced AMPA receptors. Neuron. 1995 Apr;14(4):833–843. doi: 10.1016/0896-6273(95)90227-9. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Mayer M. L. Negative allosteric modulation of wild-type and mutant AMPA receptors by GYKI 53655. Mol Pharmacol. 1996 Jan;49(1):142–148. [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Mayer M. L. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994 Jul;46(1):129–138. [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Winters C. A., Mayer M. L., Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993 Dec;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain A. V., Morales M., Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995 Jan;14(1):185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Vyklicky L., Jr, Mayer M. L. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993 Aug;13(8):3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman I. M., Trussell L. O. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992 Jul;9(1):173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y., Bettler B., Hartley M., Sheppard P. O., O'Hara P. J., Heinemann S. F. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994 Dec;13(6):1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Tarnawa I., Farkas S., Berzsenyi P., Pataki A., Andrási F. Electrophysiological studies with a 2,3-benzodiazepine muscle relaxant: GYKI 52466. Eur J Pharmacol. 1989 Aug 22;167(2):193–199. doi: 10.1016/0014-2999(89)90579-7. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding T. J., Huettner J. E. Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol Pharmacol. 1995 Mar;47(3):582–587. [PubMed] [Google Scholar]
- Wong L. A., Mayer M. L. Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993 Sep;44(3):504–510. [PubMed] [Google Scholar]
- Yamada K. A., Tang C. M. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993 Sep;13(9):3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic I., Thompson D. M., Bertolino M., Uzunov D., DiBella M., Costa E., Guidotti A. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. J Pharmacol Exp Ther. 1995 Jan;272(1):300–309. [PubMed] [Google Scholar]
- Zorumski C. F., Yamada K. A., Price M. T., Olney J. W. A benzodiazepine recognition site associated with the non-NMDA glutamate receptor. Neuron. 1993 Jan;10(1):61–67. doi: 10.1016/0896-6273(93)90242-j. [DOI] [PubMed] [Google Scholar]


