Abstract
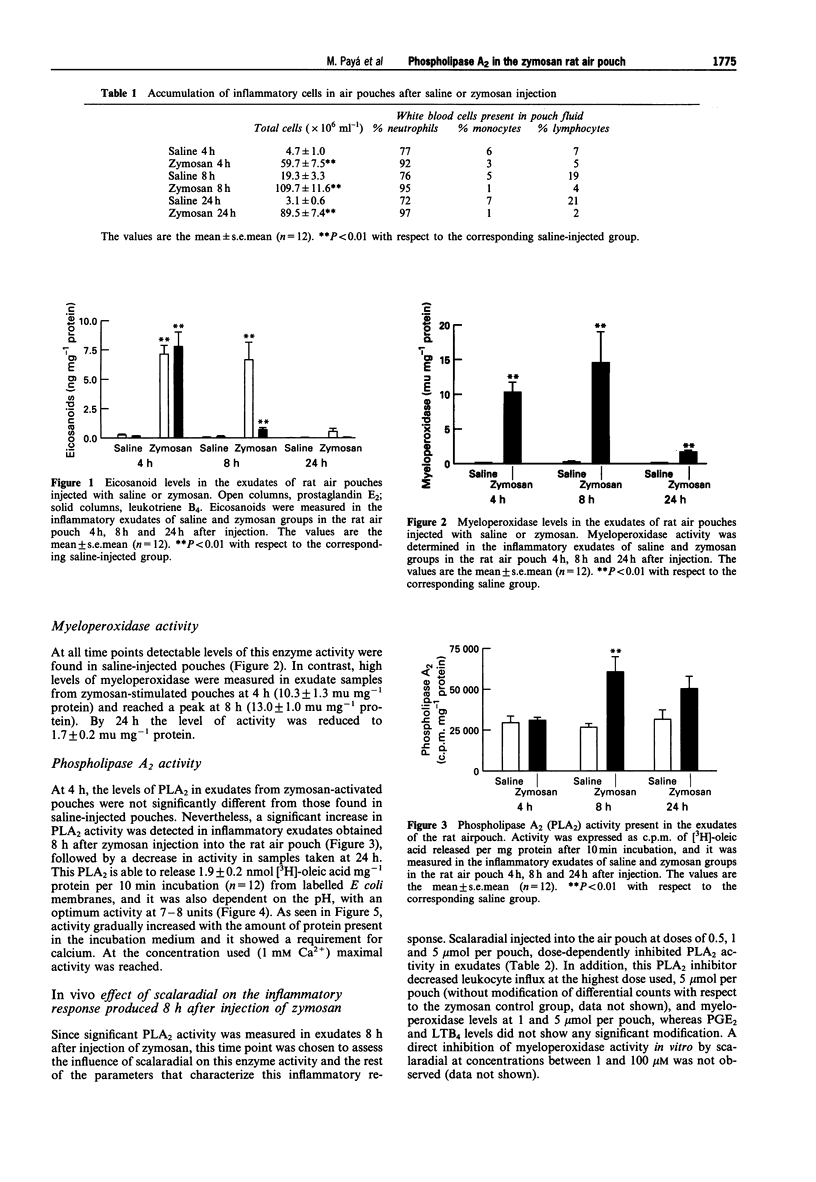
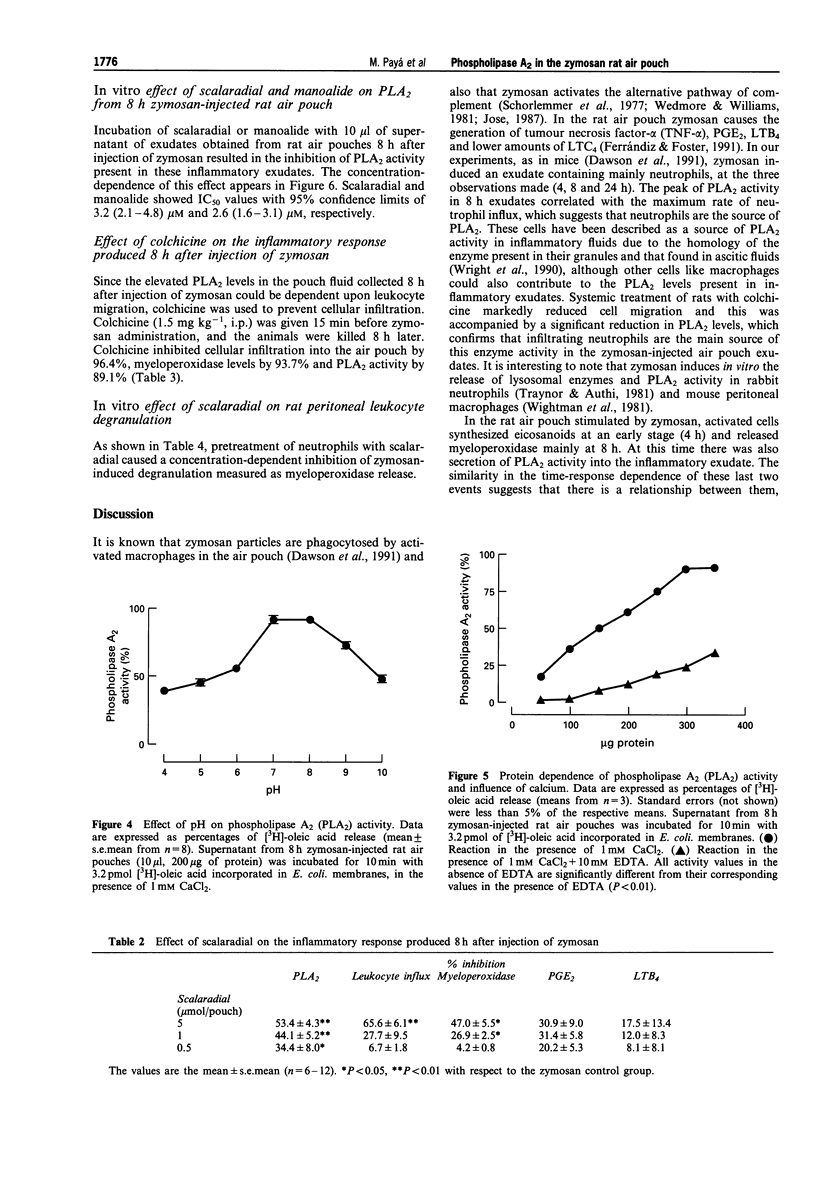
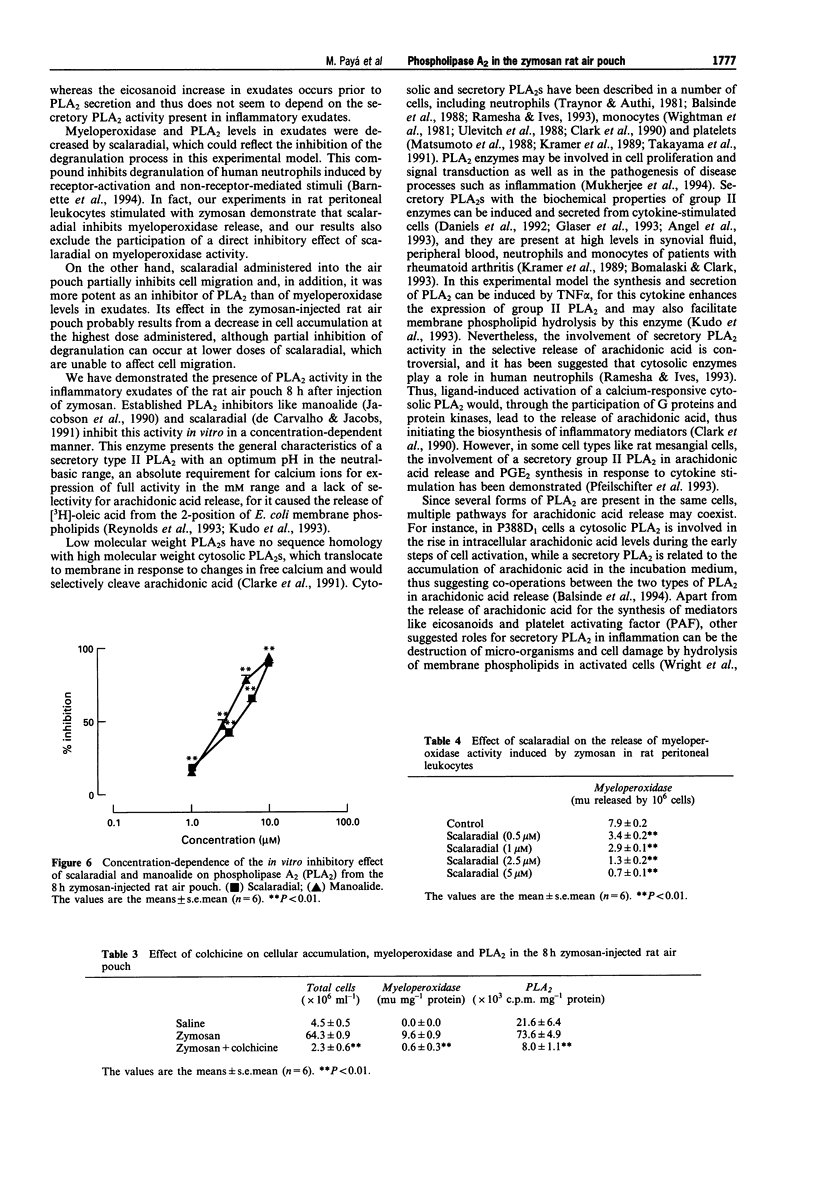
1. In the zymosan rat air pouch model of inflammation we have assessed the time dependence of phospholipase A2 (PLA2) accumulation in the inflammatory exudates as well as cell migration, myeloperoxidase activity, prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) levels. 2. A significant increase in PLA2 activity was detected in 1,200 g supernatants of exudates 8 h after injection of zymosan into rat air pouch. This event coincided with peaks in cell accumulation (mainly neutrophils) and myeloperoxidase activity in exudates and was preceded by a rise in eicosanoid levels. 3. This enzyme (without further purification) behaved as a secretory type II PLA2 with an optimum pH at 7-8 units, lack of selectivity for arachidonate release and dependence on mM calcium concentrations for maximal activity. 4. The PLA2 inhibitors manoalide and scalaradial inhibited this enzyme activity in vitro in a concentration-dependent manner. Scalaradial also inhibited zymosan stimulated myeloperoxidase release in vitro. 5. Injection of the marine PLA2 inhibitor scalaradial together with zymosan into the pouch at doses of 0.5, 1 and 5 mumol per pouch resulted in a dose-dependent inhibition of PLA2 activity in exudates collected 8 h later. Myeloperoxidase levels and cell migration were also decreased, while eicosanoid levels were not modified. 6. Colchicine administration to rats prevented infiltration and decreased PLA2 levels in the 8 h zymosan-injected air pouch. 7. These results indicate that during inflammatory response to zymosan in the rat air pouch a secretory PLA2 activity is released into the exudates. The source of this activity is mainly the neutrophil which migrates into the pouch. 8. Scalaradial exerts anti-inflammatory effects in the zymosan air pouch.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel J., Colard O., Chevy F., Fournier C. Interleukin-1-mediated phospholipid breakdown and arachidonic acid release in human synovial cells. Arthritis Rheum. 1993 Feb;36(2):158–167. doi: 10.1002/art.1780360205. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Barbour S. E., Bianco I. D., Dennis E. A. Arachidonic acid mobilization in P388D1 macrophages is controlled by two distinct Ca(2+)-dependent phospholipase A2 enzymes. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11060–11064. doi: 10.1073/pnas.91.23.11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J., Diez E., Schüller A., Mollinedo F. Phospholipase A2 activity in resting and activated human neutrophils. Substrate specificity, pH dependence, and subcellular localization. J Biol Chem. 1988 Feb 5;263(4):1929–1936. [PubMed] [Google Scholar]
- Barnette M. S., Rush J., Marshall L. A., Foley J. J., Schmidt D. B., Sarau H. M. Effects of scalaradial, a novel inhibitor of 14 kDa phospholipase A2, on human neutrophil function. Biochem Pharmacol. 1994 Apr 29;47(9):1661–1667. doi: 10.1016/0006-2952(94)90545-2. [DOI] [PubMed] [Google Scholar]
- Bomalaski J. S., Clark M. A. Phospholipase A2 and arthritis. Arthritis Rheum. 1993 Feb;36(2):190–198. doi: 10.1002/art.1780360208. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Milona N., Knopf J. L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. H., Finnen M. J., Hill M. E., Lackie J. M. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992 Jan;75(1):157–163. [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Sedgwick A. D., Edwards J. C., Lees P. A comparative study of the cellular, exudative and histological responses to carrageenan, dextran and zymosan in the mouse. Int J Tissue React. 1991;13(4):171–185. [PubMed] [Google Scholar]
- De Young L. M., Kheifets J. B., Ballaron S. J., Young J. M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989 Mar;26(3-4):335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Sundler R. Differential activation of phosphatidylinositol deacylation and a pathway via diphosphoinositide in macrophages responding to zymosan and ionophore A23187. J Biol Chem. 1984 Mar 10;259(5):3111–3116. [PubMed] [Google Scholar]
- Farina P. R., Graham A. G., Hoffman A. F., Watrous J. M., Borgeat P., Nadeau M., Hansen G., Dinallo R. M., Adams J., Miao C. K. BIRM 270: a novel inhibitor of arachidonate release that blocks leukotriene B4 and platelet-activating factor biosynthesis in human neutrophils. J Pharmacol Exp Ther. 1994 Dec;271(3):1418–1426. [PubMed] [Google Scholar]
- Ferrándiz M. L., Foster S. J. Tumour necrosis factor production in a rat airpouch model of inflammation: role of eicosanoids. Agents Actions. 1991 Mar;32(3-4):289–294. doi: 10.1007/BF01980888. [DOI] [PubMed] [Google Scholar]
- Ferrándiz M. L., Sanz M. J., Bustos G., Payá M., Alcaraz M. J., De Rosa S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur J Pharmacol. 1994 Feb 21;253(1-2):75–82. doi: 10.1016/0014-2999(94)90759-5. [DOI] [PubMed] [Google Scholar]
- Franson R., Patriarca P., Elsbach P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J Lipid Res. 1974 Jul;15(4):380–388. [PubMed] [Google Scholar]
- Glaser K. B., Mobilio D., Chang J. Y., Senko N. Phospholipase A2 enzymes: regulation and inhibition. Trends Pharmacol Sci. 1993 Mar;14(3):92–98. doi: 10.1016/0165-6147(93)90071-q. [DOI] [PubMed] [Google Scholar]
- Jacobson P. B., Marshall L. A., Sung A., Jacobs R. S. Inactivation of human synovial fluid phospholipase A2 by the marine natural product, manoalide. Biochem Pharmacol. 1990 May 15;39(10):1557–1564. doi: 10.1016/0006-2952(90)90521-l. [DOI] [PubMed] [Google Scholar]
- Jose P. J. Complement-derived peptide mediators of inflammation. Br Med Bull. 1987 Apr;43(2):336–343. doi: 10.1093/oxfordjournals.bmb.a072186. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Kudo I., Murakami M., Hara S., Inoue K. Mammalian non-pancreatic phospholipases A2. Biochim Biophys Acta. 1993 Nov 3;1170(3):217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Marshall L. A., Winkler J. D., Griswold D. E., Bolognese B., Roshak A., Sung C. M., Webb E. F., Jacobs R. Effects of scalaradial, a type II phospholipase A2 inhibitor, on human neutrophil arachidonic acid mobilization and lipid mediator formation. J Pharmacol Exp Ther. 1994 Feb;268(2):709–717. [PubMed] [Google Scholar]
- Matsumoto T., Tao W., Sha'afi R. I. Demonstration of calcium-dependent phospholipase A2 activity in membrane preparation of rabbit neutrophils. Absence of activation by fMet-Leu-Phe, phorbol 12-myristate 13-acetate and A-kinase. Biochem J. 1988 Mar 1;250(2):343–348. doi: 10.1042/bj2500343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. M., Glaser K. B., Jacobs R. S. Regulation of eicosanoid biosynthesis in vitro and in vivo by the marine natural product manoalide: a potent inactivator of venom phospholipases. J Pharmacol Exp Ther. 1988 Mar;244(3):871–878. [PubMed] [Google Scholar]
- Meagher L. C., Moonga B. S., Haslett C., Huang C. L., Zaidi M. Single pulses of cytoplasmic calcium associated with phagocytosis of individual zymosan particles by macrophages. Biochem Biophys Res Commun. 1991 May 31;177(1):460–465. doi: 10.1016/0006-291x(91)92006-6. [DOI] [PubMed] [Google Scholar]
- Miyake A., Yamamoto H., Kubota E., Hamaguchi K., Kouda A., Honda K., Kawashima H. Suppression of inflammatory responses to 12-O-tetradecanoyl-phorbol-13-acetate and carrageenin by YM-26734, a selective inhibitor of extracellular group II phospholipase A2. Br J Pharmacol. 1993 Sep;110(1):447–453. doi: 10.1111/j.1476-5381.1993.tb13831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney M. A., Alcaraz M. J., Forder R. A., Carey F., Hoult J. R. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol. 1988 Nov;40(11):787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Miele L., Pattabiraman N. Phospholipase A2 enzymes: regulation and physiological role. Biochem Pharmacol. 1994 Jul 5;48(1):1–10. doi: 10.1016/0006-2952(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Neves P. C., Neves M. C., Cruz A. B., Sant'Ana A. E., Yunes R. A., Calixto J. B. Differential effects of Mandevilla velutina compounds on paw oedema induced by phospholipase A2 and phospholipase C. Eur J Pharmacol. 1993 Oct 26;243(3):213–219. doi: 10.1016/0014-2999(93)90177-j. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Schalkwijk C., Briner V. A., van den Bosch H. Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. Its contribution to arachidonic acid release and prostaglandin synthesis by cultured rat glomerular cells. J Clin Invest. 1993 Nov;92(5):2516–2523. doi: 10.1172/JCI116860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts B. C., Faulkner D. J., Jacobs R. S. Phospholipase A2 inhibitors from marine organisms. J Nat Prod. 1992 Dec;55(12):1701–1717. doi: 10.1021/np50090a001. [DOI] [PubMed] [Google Scholar]
- Ramesha C. S., Ives D. L. Detection of arachidonoyl-selective phospholipase A2 in human neutrophil cytosol. Biochim Biophys Acta. 1993 May 20;1168(1):37–44. doi: 10.1016/0005-2760(93)90263-9. [DOI] [PubMed] [Google Scholar]
- Reynolds L. J., Hughes L. L., Louis A. I., Kramer R. M., Dennis E. A. Metal ion and salt effects on the phospholipase A2, lysophospholipase, and transacylase activities of human cytosolic phospholipase A2. Biochim Biophys Acta. 1993 Apr 23;1167(3):272–280. doi: 10.1016/0005-2760(93)90229-3. [DOI] [PubMed] [Google Scholar]
- Schorlemmer H. U., Edwards J. H., Davies P., Allison A. C. Macrophage responses to mouldy hay dust, Micropolyspora faeni and zymosan, activators of complement by the alternative pathway. Clin Exp Immunol. 1977 Feb;27(2):198–207. [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Takayama K., Kudo I., Kim D. K., Nagata K., Nozawa Y., Inoue K. Purification and characterization of human platelet phospholipase A2 which preferentially hydrolyzes an arachidonoyl residue. FEBS Lett. 1991 May 6;282(2):326–330. doi: 10.1016/0014-5793(91)80506-x. [DOI] [PubMed] [Google Scholar]
- Tramposch K. M., Chilton F. H., Stanley P. L., Franson R. C., Havens M. B., Nettleton D. O., Davern L. B., Darling I. M., Bonney R. J. Inhibitor of phospholipase A2 blocks eicosanoid and platelet activating factor biosynthesis and has topical anti-inflammatory activity. J Pharmacol Exp Ther. 1994 Nov;271(2):852–859. [PubMed] [Google Scholar]
- Traynor J. R., Authi K. S. Phospholipase A2 activity of lysosomal origin secreted by polymorphonuclear leucocytes during phagocytosis or on treatment with calcium. Biochim Biophys Acta. 1981 Sep 24;665(3):571–577. doi: 10.1016/0005-2760(81)90272-1. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Watanabe Y., Sano M., Lister M. D., Deems R. A., Dennis E. A. Solubilization, purification, and characterization of a membrane-bound phospholipase A2 from the P388D1 macrophage-like cell line. J Biol Chem. 1988 Mar 5;263(7):3079–3085. [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986 Oct;55(4):391–404. [PubMed] [Google Scholar]
- Vishwanath B. S., Fawzy A. A., Franson R. C. Edema-inducing activity of phospholipase A2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation. 1988 Dec;12(6):549–561. doi: 10.1007/BF00914317. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Weiss J., Inada M., Elsbach P., Crowl R. M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994 Oct 21;269(42):26331–26337. [PubMed] [Google Scholar]
- Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The selective release of phospholipase A2 by resident mouse peritoneal macrophages. Biochem J. 1981 Nov 15;200(2):441–444. doi: 10.1042/bj2000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. W., Ooi C. E., Weiss J., Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990 Apr 25;265(12):6675–6681. [PubMed] [Google Scholar]
- de Carvalho M. S., Jacobs R. S. Two-step inactivation of bee venom phospholipase A2 by scalaradial. Biochem Pharmacol. 1991 Sep 27;42(8):1621–1626. doi: 10.1016/0006-2952(91)90432-5. [DOI] [PubMed] [Google Scholar]



