Abstract
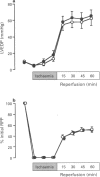
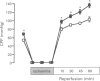
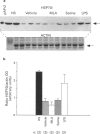
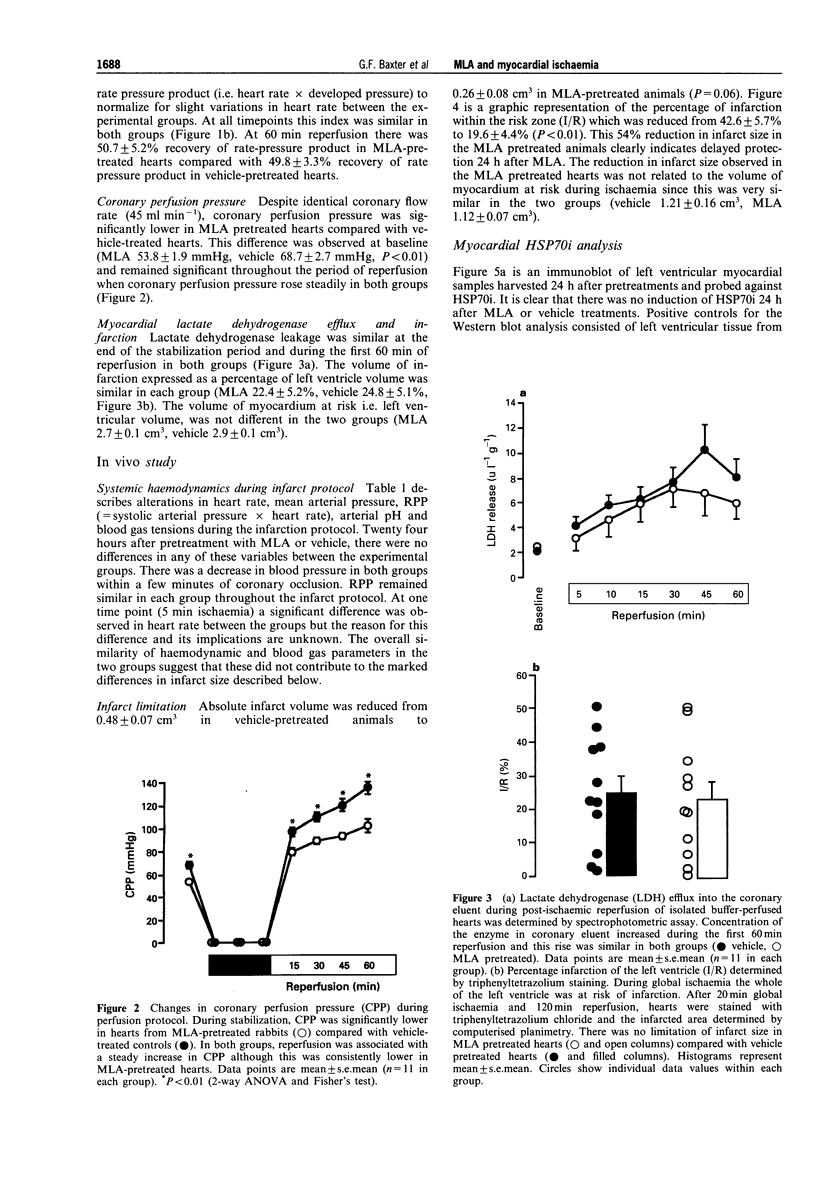
1. Monophosphoryl lipid A (MLA) is a non-pyrogenic derivative of Salmonella lipopolysaccharide. Administration of this agent at high doses to rats and at low doses to dogs was previously shown to confer marked protection against ischaemia-reperfusion 24 h later, although the cellular mechanisms of this delayed protection are obscure. We hypothesized that MLA pretreatment causes the induction of the 70 kDa cytoprotective stress protein HSP70i in the myocardium. If this were the case, protection against ischaemia-reperfusion injury would be observed both in vitro and in vivo. 2. Rabbits were pretreated with MLA 0.035 mg kg-1, i.v. or vehicle solution. For the in vitro study, hearts were isolated 24 h later and Langendorff-perfused with Krebs-Henseleit buffer at 37 degrees C. Global ischaemia was induced for 20 min followed by 120 min reperfusion. Recovery of post-ischaemic left ventricular function and lactate dehydrogenase efflux was similar in MLA and vehicle pretreated hearts and there was no significant difference in the percentage of infarction of the left ventricle determined by triphenyltetrazolium staining (MLA 22.4 +/- 5.2%, vehicle 24.8 +/- 5.1%). 3. When 30 min regional ischaemia and 120 min reperfusion was instituted in pentobarbitone-anaesthetized rabbits 24 h after pretreatment with MLA or vehicle, the percentage infarction within the risk zone was reduced from 42.6 +/- 5.7% in vehicle pretreated animals to 19.6 +/- 4.4% in MLA pretreated animals (P < 0.01). 4. Determination of myocardial HSP70i content by Western blot analysis showed that MLA treatment did not increase HSP70i immunoreactivity. 5. We conclude that MLA at this dose confers protection only against ischaemia-reperfusion injury in vivo and that this protection is not related to induction of HSP70i. Because protection was observed only in vivo it seems possible that the delayed protection conferred by MLA is mediated by effects on humoral or blood-borne factors.
Full text
PDF

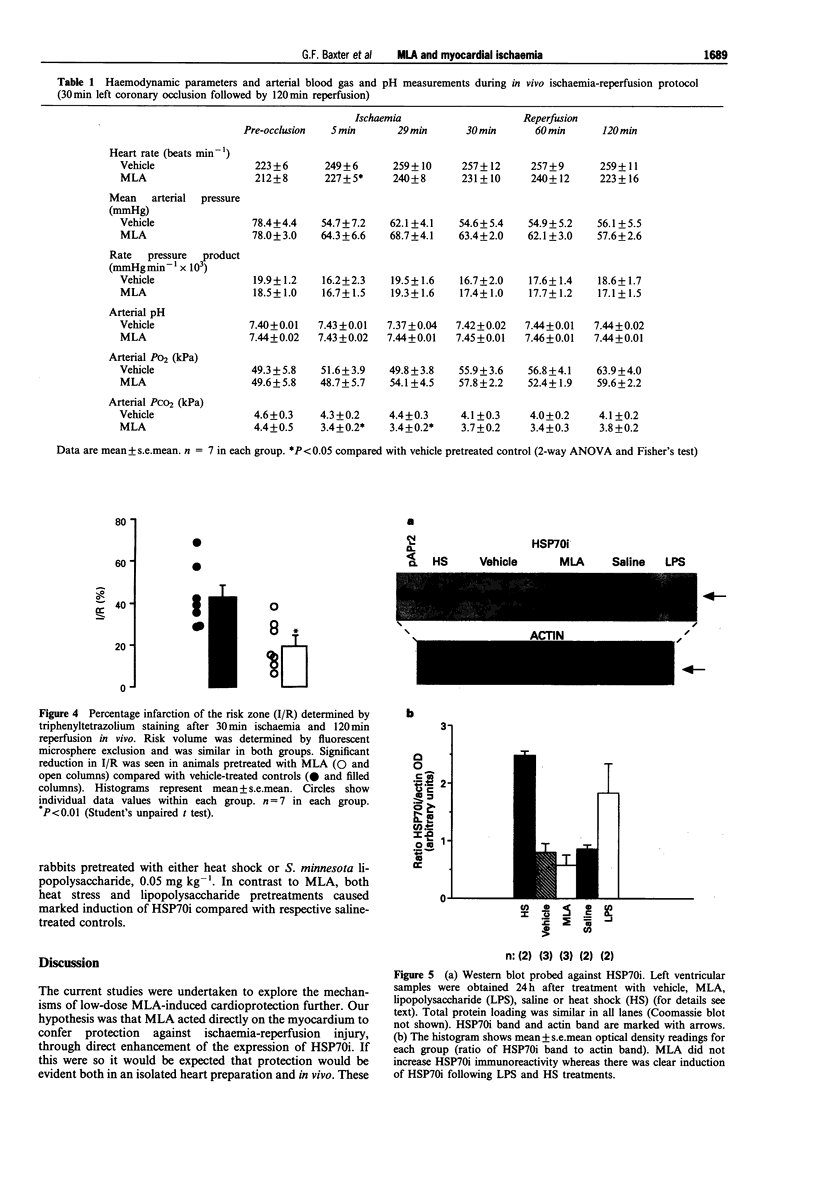



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter G. F., Marber M. S., Patel V. C., Yellon D. M. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994 Dec;90(6):2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Anderson B. O., Repine J. E., Shanley P. F., White C. W., Grosso M. A., Banerjee A., Bensard D. D., Harken A. H. Neutrophils contribute to TNF induced myocardial tolerance to ischaemia. J Mol Cell Cardiol. 1992 May;24(5):485–495. doi: 10.1016/0022-2828(92)91838-v. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Grosso M. A., Terada L. S., Whitman G. J., Banerjee A., White C. W., Harken A. H., Repine J. E. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie R. W., Karmazyn M., Kloc M., Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988 Sep;63(3):543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Tanguay R. M., Kingma J. G., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993 Mar;87(3):963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Mehta H. B., Barrieux A., Guth B. D., Neeley W. E., Ross J., Jr Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ Res. 1986 Jul;59(1):110–114. doi: 10.1161/01.res.59.1.110. [DOI] [PubMed] [Google Scholar]
- Donati Y. R., Slosman D. O., Polla B. S. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990 Dec 15;40(12):2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- Dougall W. C., Nick H. S. Manganese superoxide dismutase: a hepatic acute phase protein regulated by interleukin-6 and glucocorticoids. Endocrinology. 1991 Nov;129(5):2376–2384. doi: 10.1210/endo-129-5-2376. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Meerbaum S., Rit J., Lando U., Kanmatsuse K., Mercier J. C., Corday E., Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981 May;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Frank L., Summerville J., Massaro D. Potection from oxygen toxicity with endotoxin. Role of the endogenous antioxidant enzymes of the lung. J Clin Invest. 1980 May;65(5):1104–1110. doi: 10.1172/JCI109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P. R. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995 Mar 15;91(6):1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- Heads R. J., Yellon D. M., Latchman D. S. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995 Aug;27(8):1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Garlick P. B. Species variation in myocardial anoxic enzyme release, glucose protection and reoxygenation damage. J Mol Cell Cardiol. 1976 Apr;8(4):329–339. doi: 10.1016/0022-2828(76)90007-9. [DOI] [PubMed] [Google Scholar]
- Hoshida S., Kuzuya T., Fuji H., Yamashita N., Oe H., Hori M., Suzuki K., Taniguchi N., Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. Am J Physiol. 1993 Jan;264(1 Pt 2):H33–H39. doi: 10.1152/ajpheart.1993.264.1.H33. [DOI] [PubMed] [Google Scholar]
- Hutter M. M., Sievers R. E., Barbosa V., Wolfe C. L. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994 Jan;89(1):355–360. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- Jenkins D. P., Pugsley W. B., Yellon D. M. Ischaemic preconditioning in a model of global ischaemia: infarct size limitation, but no reduction of stunning. J Mol Cell Cardiol. 1995 Aug;27(8):1623–1632. doi: 10.1016/s0022-2828(95)90590-1. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Mailer K., Currie R. W. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990 Aug;259(2 Pt 2):H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R., Werns S. W., Fantone J. C. The role of the neutrophil and free radicals in ischemic myocardial injury. J Mol Cell Cardiol. 1989 Dec;21(12):1241–1251. doi: 10.1016/0022-2828(89)90670-6. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Latchman D. S., Walker J. M., Yellon D. M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993 Sep;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Mestril R., Chi S. H., Sayen M. R., Yellon D. M., Dillmann W. H. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995 Apr;95(4):1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R., Chi S. H., Sayen M. R., O'Reilly K., Dillmann W. H. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest. 1994 Feb;93(2):759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Nelson D. W., Brown J. M., Banerjee A., Bensard D. D., Rogers K. B., Locke-Winter C. R., Anderson B. O., Harken A. H. Pretreatment with a nontoxic derivative of endotoxin induces functional protection against cardiac ischemia/reperfusion injury. Surgery. 1991 Aug;110(2):365–369. [PubMed] [Google Scholar]
- Plumier J. C., Ross B. M., Currie R. W., Angelidis C. E., Kazlaris H., Kollias G., Pagoulatos G. N. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995 Apr;95(4):1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Murry C. E., Richard V. J. The role of neutrophils and free radicals in the ischemic-reperfused heart: why the confusion and controversy? J Mol Cell Cardiol. 1989 Dec;21(12):1225–1239. doi: 10.1016/0022-2828(89)90669-x. [DOI] [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Song W., Furman B. L., Parratt J. R. Attenuation by dexamethasone of endotoxin protection against ischaemia-induced ventricular arrhythmias. Br J Pharmacol. 1994 Dec;113(4):1083–1084. doi: 10.1111/j.1476-5381.1994.tb17105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steare S. E., Yellon D. M. Increased endogenous catalase activity caused by heat stress does not protect the isolated rat heart against exogenous hydrogen peroxide. Cardiovasc Res. 1994 Jul;28(7):1096–1101. doi: 10.1093/cvr/28.7.1096. [DOI] [PubMed] [Google Scholar]
- Yao Z., Auchampach J. A., Pieper G. M., Gross G. J. Cardioprotective effects of monophosphoryl lipid A, a novel endotoxin analogue, in the dog. Cardiovasc Res. 1993 May;27(5):832–838. doi: 10.1093/cvr/27.5.832. [DOI] [PubMed] [Google Scholar]
- Yao Z., Rasmussen J. L., Hirt J. L., Mei D. A., Pieper G. M., Gross G. J. Effects of monophosphoryl lipid A on myocardial ischemia/reperfusion injury in dogs. J Cardiovasc Pharmacol. 1993 Oct;22(4):653–663. doi: 10.1097/00005344-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Ytrehus K., Liu Y., Tsuchida A., Miura T., Liu G. S., Yang X. M., Herbert D., Cohen M. V., Downey J. M. Rat and rabbit heart infarction: effects of anesthesia, perfusate, risk zone, and method of infarct sizing. Am J Physiol. 1994 Dec;267(6 Pt 2):H2383–H2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]