Abstract
1. The adult respiratory distress syndrome (ARDS) is an acute lung inflammation developed after direct or indirect contact with pathogenic agents. In the present study, a mouse model was developed to mimic this condition using aerosolized bacterial lipopolysaccharide (LPS) and to investigate the mechanisms involved in the lung inflammatory response. 2. Inhalation of LPS led to a time and dose-dependent increase in tumour necrosis factor-alpha (TNF-alpha) production and neutrophil recruitment into the bronchoalveolar lavage fluid (BALF) of Balb/c mice. Under the same conditions, neutrophil infiltration was also found in the BALF of the LPS-sensitive mouse strain C3H/HeN, but was absent in the LPS-resistant strain C3H/HeJ. Intranasal administration of murine recombinant TNF-alpha also triggered neutrophil recruitment. 3. One hour after inhalation of LPS, half of the maximal level of TNF-alpha was measured in the BALF, but only a few neutrophils were detected at this time. The peak TNF-alpha concentration was reached at 3 h, when the neutrophil amount started to increase. At 24 h, maximal neutrophil number was found in the BALF and TNF-alpha was no longer present. 4. Pretreatment of mice under different experimental conditions demonstrated that: (a) cycloheximide almost completely blocks both neutrophil recruitment and TNF-alpha production; (b) anti TNF-alpha antibodies block neutrophil recruitment; (c) indomethacin or aspirin enhance by two fold neutrophil recruitment; (d) indomethacin significantly increases TNF-alpha production 1 h after inhalation of LPS; (e) dibutyryl cyclic AMP and prostaglandin E2 (PGE2) block both neutrophil recruitment and TNF-alpha production. 5. It is concluded that aerosolized LPS in mice triggers an acute lung inflammation which can be used as a potential model of inhalational ARDS and that, strategies leading to the elevation of cyclic AMP levels in vivo can be effective in modulating LPS-induced TNF-alpha synthesis and neutrophil recruitment.
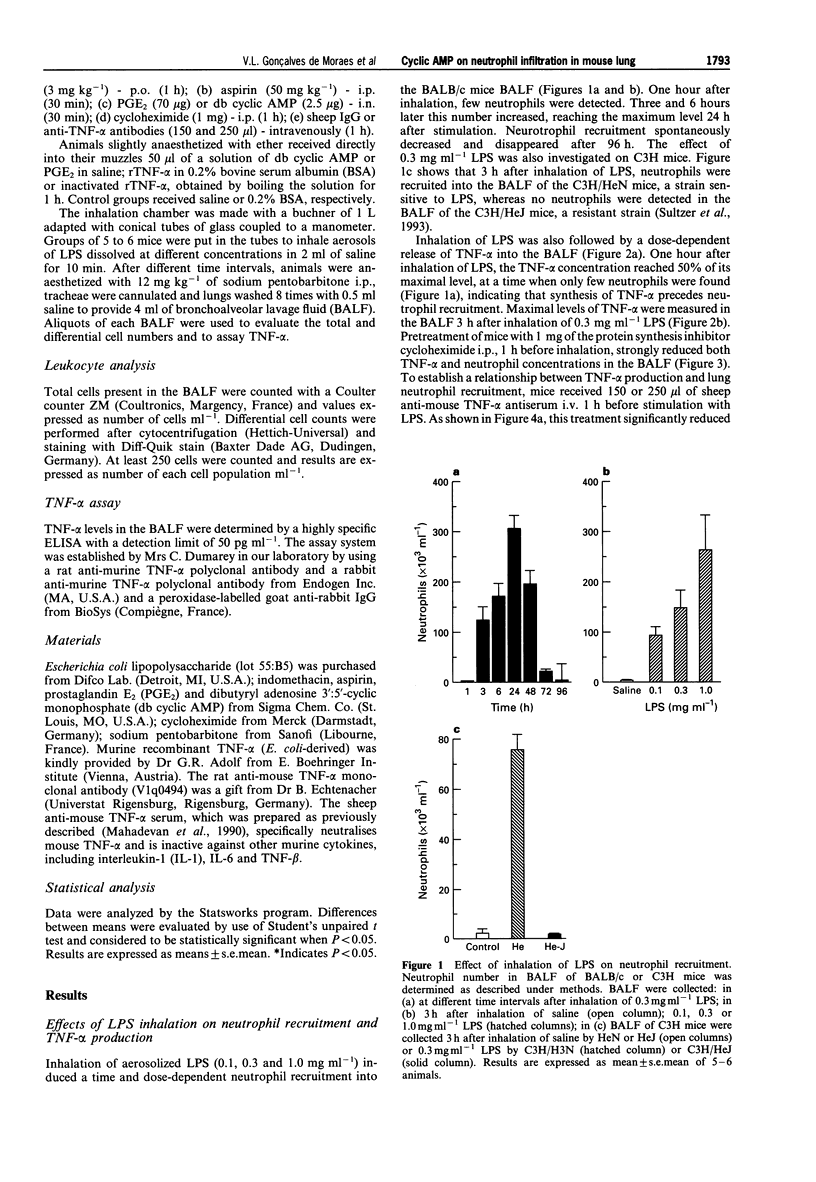
Full text
PDF
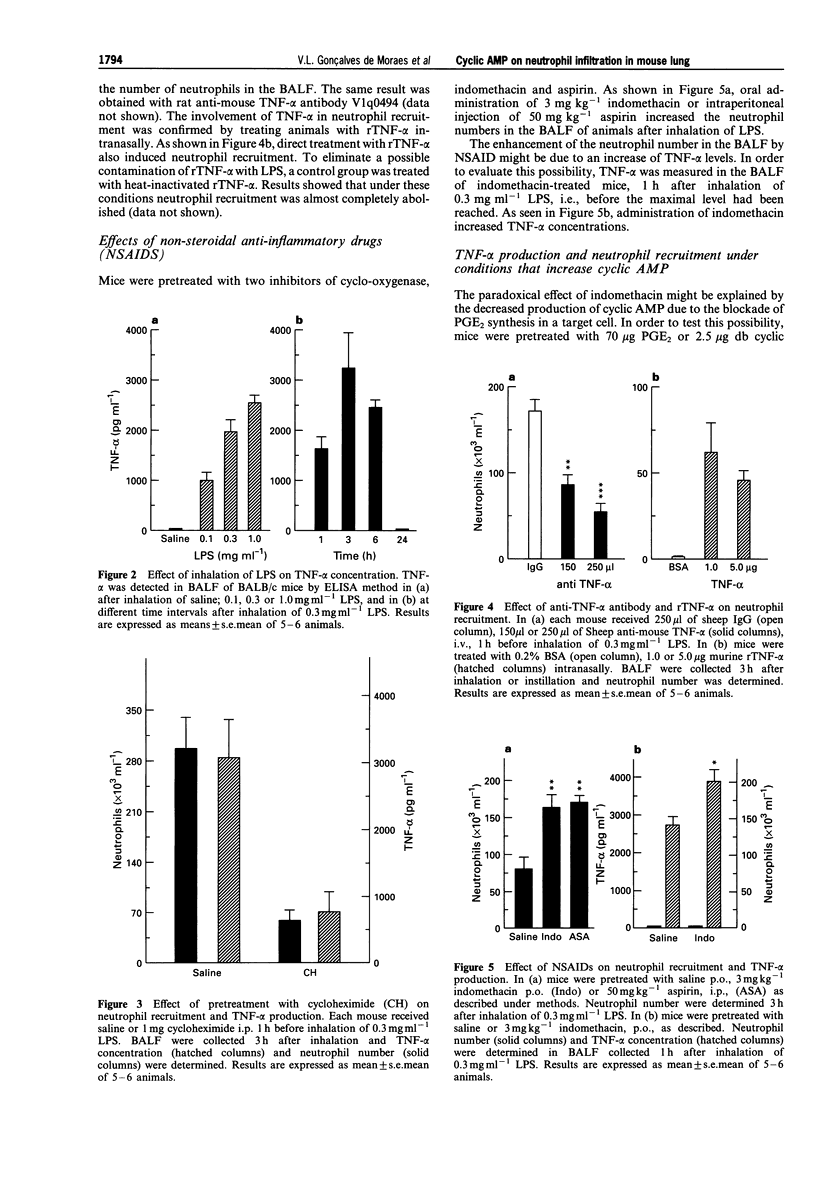
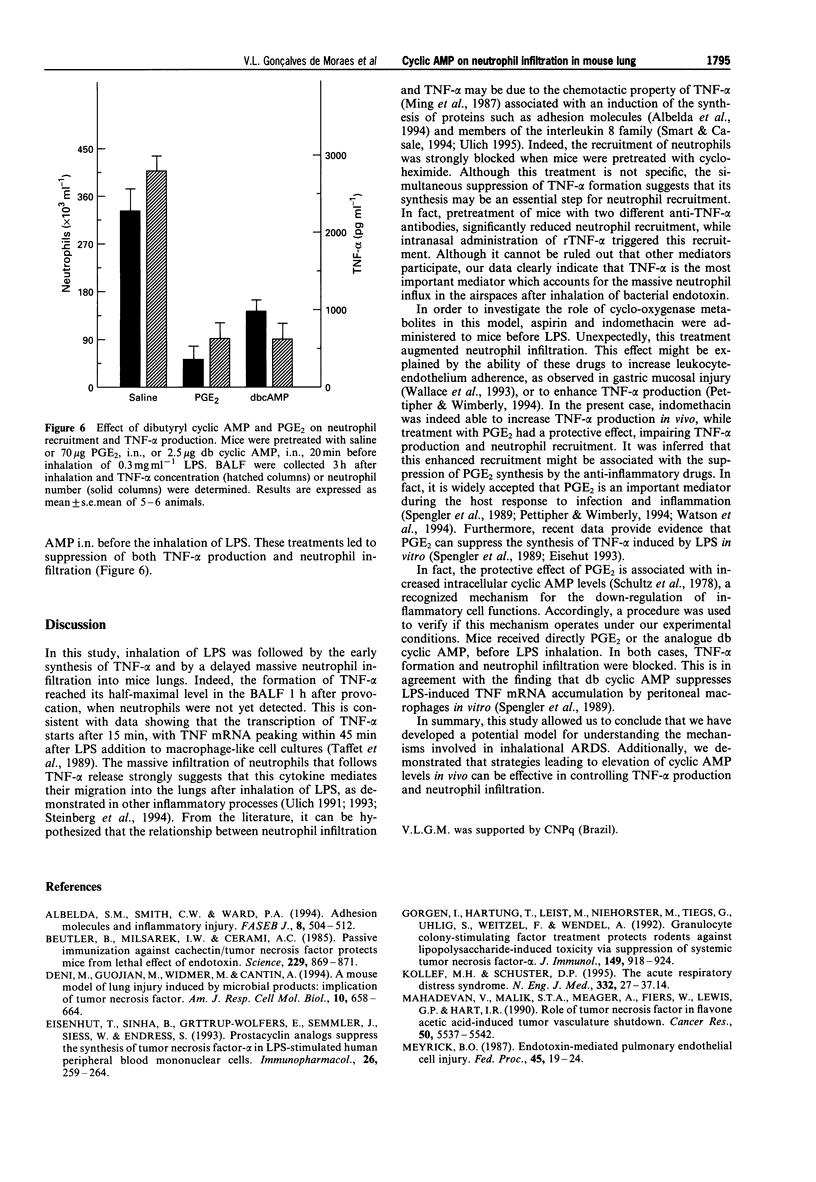

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Smith C. W., Ward P. A. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–512. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Denis M., Guojian L., Widmer M., Cantin A. A mouse model of lung injury induced by microbial products: implication of tumor necrosis factor. Am J Respir Cell Mol Biol. 1994 Jun;10(6):658–664. doi: 10.1165/ajrcmb.10.6.8003342. [DOI] [PubMed] [Google Scholar]
- Eisenhut T., Sinha B., Gröttrup-Wolfers E., Semmler J., Siess W., Endres S. Prostacyclin analogs suppress the synthesis of tumor necrosis factor-alpha in LPS-stimulated human peripheral blood mononuclear cells. Immunopharmacology. 1993 Nov-Dec;26(3):259–264. doi: 10.1016/0162-3109(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Görgen I., Hartung T., Leist M., Niehörster M., Tiegs G., Uhlig S., Weitzel F., Wendel A. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992 Aug 1;149(3):918–924. [PubMed] [Google Scholar]
- Kollef M. H., Schuster D. P. The acute respiratory distress syndrome. N Engl J Med. 1995 Jan 5;332(1):27–37. doi: 10.1056/NEJM199501053320106. [DOI] [PubMed] [Google Scholar]
- Mahadevan V., Malik S. T., Meager A., Fiers W., Lewis G. P., Hart I. R. Role of tumor necrosis factor in flavone acetic acid-induced tumor vasculature shutdown. Cancer Res. 1990 Sep 1;50(17):5537–5542. [PubMed] [Google Scholar]
- Meyrick B. O. Endotoxin-mediated pulmonary endothelial cell injury. Fed Proc. 1986 Jan;45(1):19–24. [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Pettipher E. R., Wimberly D. J. Cyclooxygenase inhibitors enhance tumour necrosis factor production and mortality in murine endotoxic shock. Cytokine. 1994 Sep;6(5):500–503. doi: 10.1016/1043-4666(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Smart S. J., Casale T. B. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994 Mar;266(3 Pt 1):L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- Spengler R. N., Spengler M. L., Lincoln P., Remick D. G., Strieter R. M., Kunkel S. L. Dynamics of dibutyryl cyclic AMP- and prostaglandin E2-mediated suppression of lipopolysaccharide-induced tumor necrosis factor alpha gene expression. Infect Immun. 1989 Sep;57(9):2837–2841. doi: 10.1128/iai.57.9.2837-2841.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg K. P., Milberg J. A., Martin T. R., Maunder R. J., Cockrill B. A., Hudson L. D. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994 Jul;150(1):113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Castagna R., Bandekar J., Wong P. Lipopolysaccharide nonresponder cells: the C3H/HeJ defect. Immunobiology. 1993 Apr;187(3-5):257–271. doi: 10.1016/S0171-2985(11)80343-8. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Singhel K. J., Overholtzer J. F., Shurtleff S. A. Regulation of tumor necrosis factor expression in a macrophage-like cell line by lipopolysaccharide and cyclic AMP. Cell Immunol. 1989 May;120(2):291–300. doi: 10.1016/0008-8749(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994 Feb;6(1):125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Howard S. C., Remick D. G., Wittwer A., Yi E. S., Yin S., Guo K., Welply J. K., Williams J. H. Intratracheal administration of endotoxin and cytokines. VI. Antiserum to CINC inhibits acute inflammation. Am J Physiol. 1995 Feb;268(2 Pt 1):L245–L250. doi: 10.1152/ajplung.1995.268.2.L245. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Watson L. R., Yin S. M., Guo K. Z., Wang P., Thang H., del Castillo J. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol. 1991 Jun;138(6):1485–1496. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Yin S., Remick D. G., Russell D., Eisenberg S. P., Kohno T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol. 1993 May;142(5):1335–1338. [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., McKnight W., Miyasaka M., Tamatani T., Paulson J., Anderson D. C., Granger D. N., Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993 Nov;265(5 Pt 1):G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- Watson R. W., Redmond H. P., Bouchier-Hayes D. Role of endotoxin in mononuclear phagocyte-mediated inflammatory responses. J Leukoc Biol. 1994 Jul;56(1):95–103. doi: 10.1002/jlb.56.1.95. [DOI] [PubMed] [Google Scholar]



