Abstract
1. This study tested the hypothesis that a nitric oxide synthase (NOS) was activated in guinea-pig ileum in vitro in response to substance P (SP), and attempted to characterize the tachykinin receptor involved in this activation by the use of selective receptor agonists and antagonists. 2. Strips of guinea-pig ileum (8 x 2 mm) were superfused (Krebs, 37 degrees C, 2 ml min-1) with: (i) tachykinin receptor agonists: SP, GR 73,632 (NK1), GR 64,349 (NK2), senktide (NK3), and neuropeptide (NP) gamma; (ii) tachykinin receptor antagonists: CP 99,994 (NK1), SR 48,968 (NK2), SR 142,801 (NK3); (iii) nerve-related agents: carbachol (CCh), atropine, tetrodotoxin (TTX), hexamethonium; (iv) NOS inhibitors: N omega-nitro-L-arginine-methyl-ester (L-NAME), N omega-monomethyl-L-arginine (L-NMMA) and aminoguanidine (AG); (v) NO-related agents, L-arginine (L-Arg), D-arginine (D-Arg), sodium nitroprusside (NaNP) and methaemoglobin. Muscle contractility was recorded isometrically and quantified as integrated area of activity. 3. SP, tachykinin receptor agonists and NP gamma (10 pM to 10 microM), produced concentration-dependent contractions of ileal strips, with EC50s in the nanomolar range, and maximal responses (Emax) attained at 0.1 microM for SP and 1 microM for the other agonists. The Emax response to SP equalled that to KCl (60 mM) taken as a 100% control (99.3% [93.0-105.7]; mean and 95% CI; n = 12); a comparable Emax contraction was obtained with the other tachykinin receptor agonists (1 microM) as well as with CCh (1 microM). 4. Under baseline conditions, L-NAME (1 microM), L-NMMA (1 microM) and AG (1 microM), failed to contract the muscle strip. In contrast, when superfused for 3 min, 10 min after SP (0.1 microM), they induced a transient contraction of the strip (e.g. for 1 microM L-NAME: 50 to 70 s duration; amplitude 73 +/- 12%, n = 24). 5. The NOS inhibitor-induced contractile response was not obtained after KCl (60 mM), GR 73,632, GR 64,349, senktide or CCh (all up to 1 microM). In contrast, this contractile response was obtained after NP gamma (1 microM). 6. Blockade of tachykinin NK1, NK2 and NK3 receptors by continuous superfusion of CP 99,994, SR 48,968 and SR 142,801 (1 microM) respectively, starting 5 min before SP, did not modify the response to L-NAME, superfused 10 min after SP (0.1 microM). The contractile response to L-NAME (1 microM) was blocked by atropine (1 microM), superfused either before or after SP. In contrast, it persisted after TTX or hexamethonium (1 microM) superfused in the same conditions. 7. The amplitude of NOS inhibitor-induced contraction (1 microM) was dependent on the concentration of priming SP (1 pM to 1 microM). In contrast, the contractile response to NOS inhibitors (1 nM to 10 microM) of the ileum strip primed with SP (0.1 microM) was not concentration-related. 8. L-NAME-induced contraction was prevented by continuous superfusion of L-Arg (1 microM), but not D-Arg (1 microM). In addition, the NO donor, sodium nitroprusside (1 microM) and the NO scavenger, methaemoglobin (10 micrograms ml-1), both prevented the contractile response to L-NAME. 9. In summary, SP and to a lesser extent NP gamma, exert a permissive action allowing contractile stimulating effects of L-NAME, L-NMMA and AG, in guinea-pig ileum in vitro, by a mechanism which apparently does not involve tachykinin NK1, NK2 and NK3 receptors. This action is likely to result from the activation of a NO-synthase by SP in the vicinity of intestinal myocytes. Thus, L-NAME, L-NMMA or AG, by blocking this SP-induced NO production, unveiled a smooth muscle contraction which involves a cholinoceptor (atropine-sensitive) mechanism.
Full text
PDF


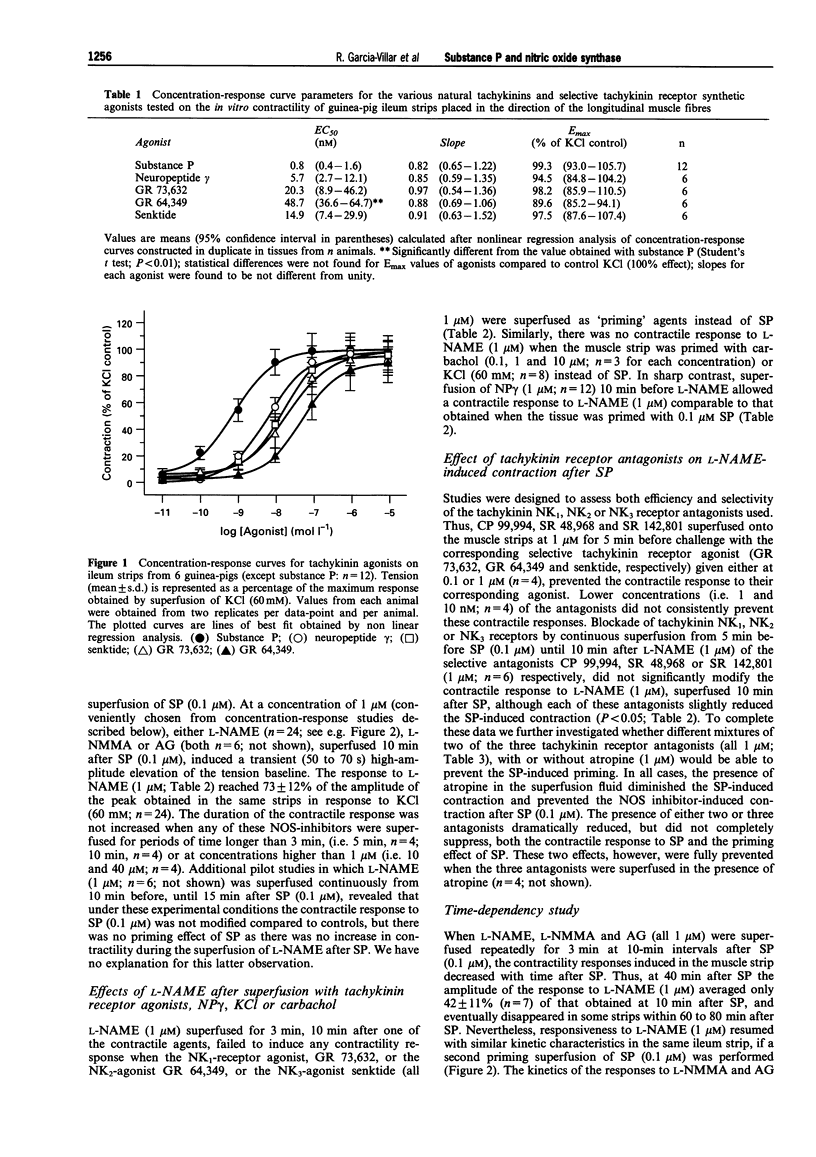
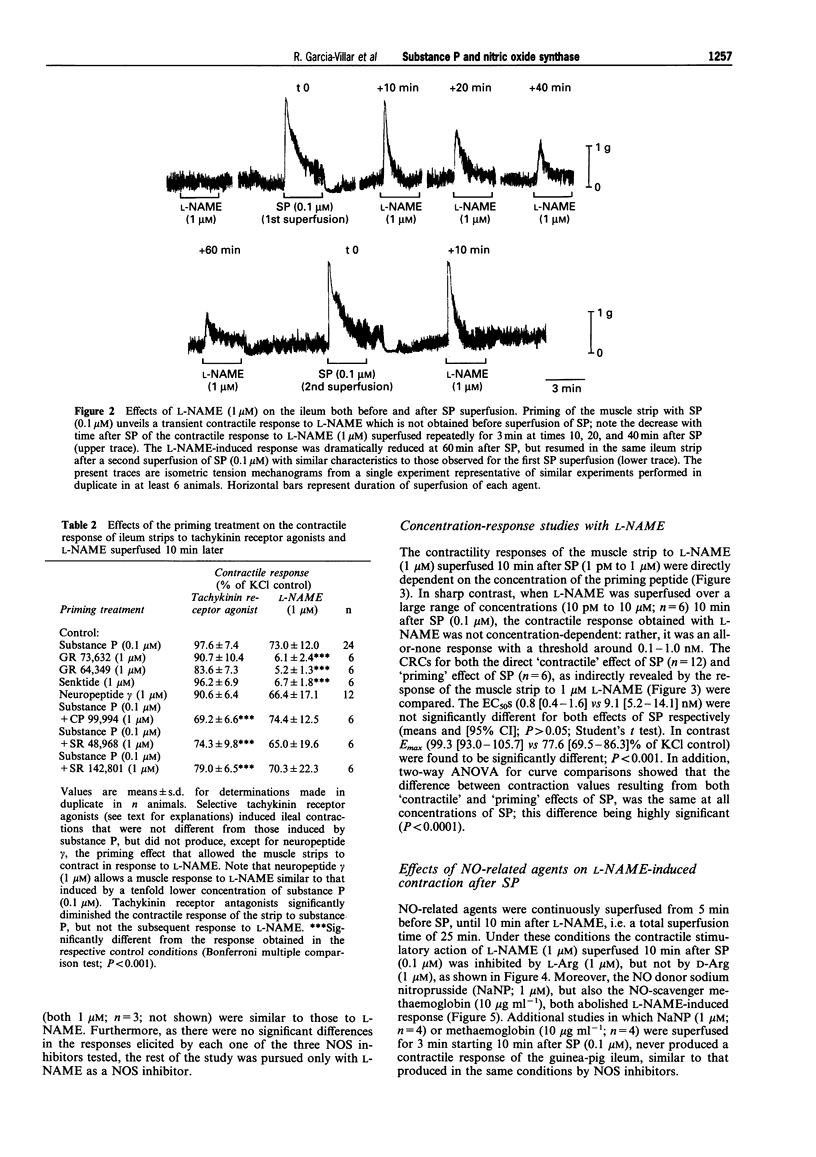
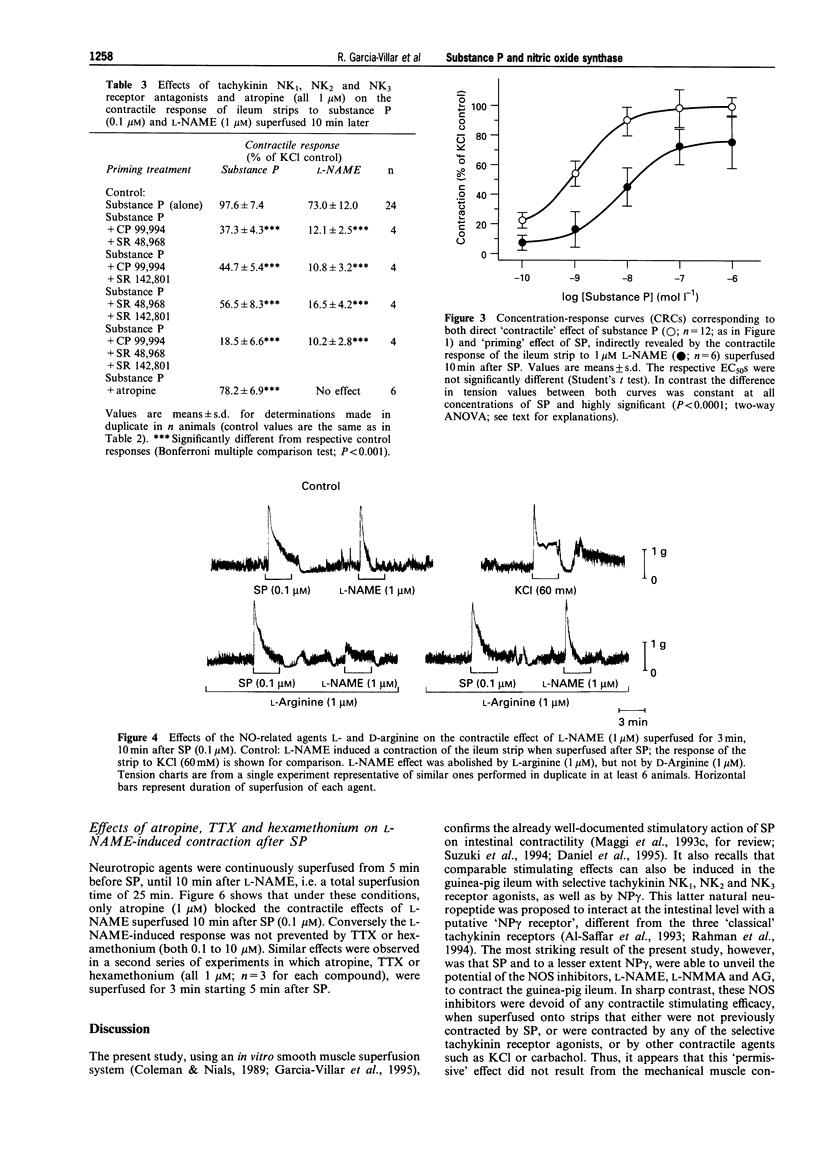
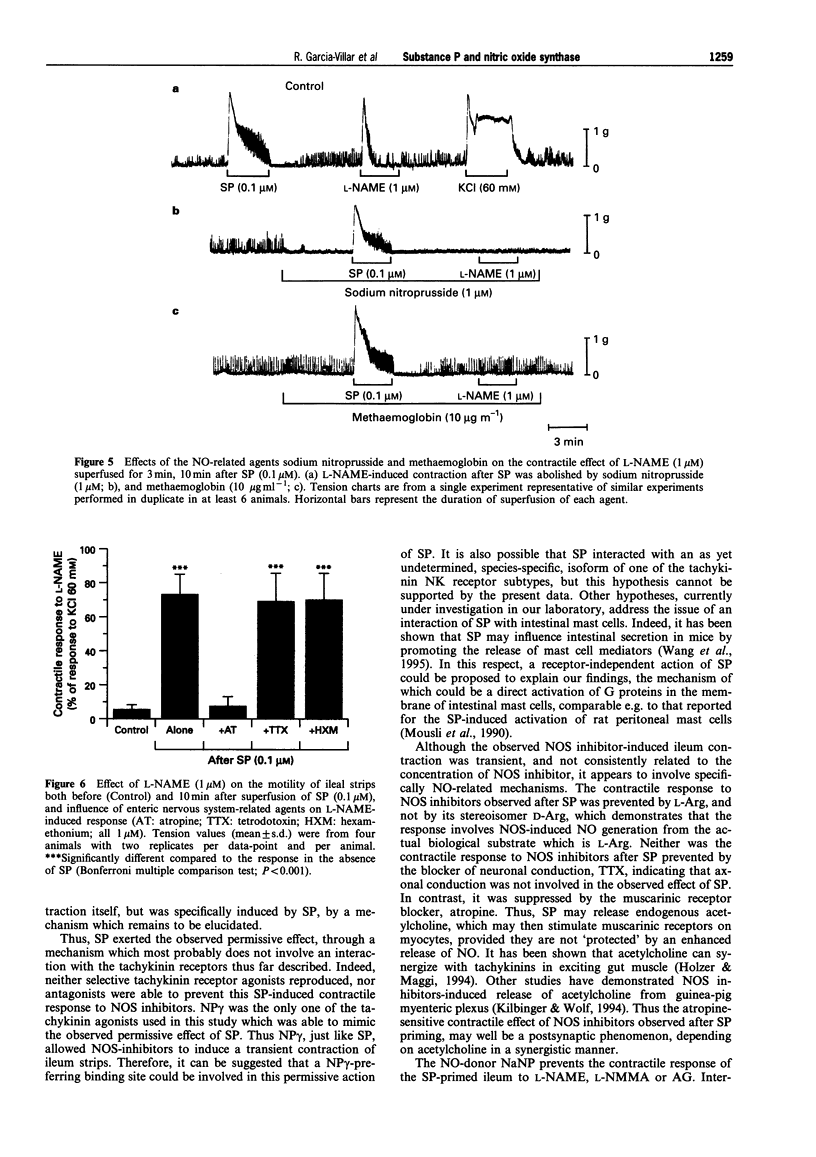


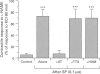
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeckxstaens G. E., Pelckmans P. A., Bogers J. J., Bult H., De Man J. G., Oosterbosch L., Herman A. G., Van Maercke Y. M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991 Feb;256(2):441–447. [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Calignano A., Whittle B. J., Di Rosa M., Moncada S. Involvement of endogenous nitric oxide in the regulation of rat intestinal motility in vivo. Eur J Pharmacol. 1992 Dec 15;229(2-3):273–276. doi: 10.1016/0014-2999(92)90567-n. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Nials A. T. Novel and versatile superfusion system. Its use in the evaluation of some spasmogenic and spasmolytic agents using guinea-pig isolated tracheal smooth muscle. J Pharmacol Methods. 1989 Mar;21(1):71–86. doi: 10.1016/0160-5402(89)90023-5. [DOI] [PubMed] [Google Scholar]
- D'Amato M., Currò D., Montuschi P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J Auton Nerv Syst. 1992 Mar;37(3):175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Parrish M. B., Watson E. G., Fox-Threlkeld J. E., Regoli D., Rainsford K. D. The tachykinin receptors inducing contractile responses of canine ileum circular muscle. Am J Physiol. 1995 Jan;268(1 Pt 1):G161–G170. doi: 10.1152/ajpgi.1995.268.1.G161. [DOI] [PubMed] [Google Scholar]
- Emonds-Alt X., Advenier C., Croci T., Manara L., Neliat G., Poncelet M., Proietto V., Santucci V., Soubrié P., Van Broeck D. SR 48968, a neurokinin A (NK2) receptor antagonist. Regul Pept. 1993 Jul 2;46(1-2):31–36. doi: 10.1016/0167-0115(93)90008-v. [DOI] [PubMed] [Google Scholar]
- Emonds-Alt X., Bichon D., Ducoux J. P., Heaulme M., Miloux B., Poncelet M., Proietto V., Van Broeck D., Vilain P., Neliat G. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56(1):PL27–PL32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- Emonds-Alt X., Vilain P., Goulaouic P., Proietto V., Van Broeck D., Advenier C., Naline E., Neliat G., Le Fur G., Brelière J. C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50(15):PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Daniel E. E. Substance P: a potent inhibitor of the canine small intestine in vivo. Am J Physiol. 1986 Jan;250(1 Pt 1):G21–G27. doi: 10.1152/ajpgi.1986.250.1.G21. [DOI] [PubMed] [Google Scholar]
- Galmiche A., Rozé C., Scarpignato C., Galmiche J. P. Monoxyde d'azote (NO), médiateur des influences non-adrénergiques non-cholinergiques du système nerveux entérique, et motricité oeso-gastrique. Gastroenterol Clin Biol. 1995 Jan;19(1):36–49. [PubMed] [Google Scholar]
- Garcia-Villar R., Green L. R., Jenkins S. L., Wentworth R. A., Coleman R. A., Nathanielsz P. W. Evidence for the presence of AH 13205-sensitive EP2-prostanoid receptors in the pregnant baboon but not in the pregnant sheep myometrium near term. J Soc Gynecol Investig. 1995 Jan-Feb;2(1):6–12. [PubMed] [Google Scholar]
- Guard S., Watson S. P., Maggio J. E., Too H. P., Watling K. J. Pharmacological analysis of [3H]-senktide binding to NK3 tachykinin receptors in guinea-pig ileum longitudinal muscle-myenteric plexus and cerebral cortex membranes. Br J Pharmacol. 1990 Apr;99(4):767–773. doi: 10.1111/j.1476-5381.1990.tb13004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L. E., Wiklund C. U., Wiklund N. P., Persson M. G., Moncada S. Modulation of autonomic neuroeffector transmission by nitric oxide in guinea pig ileum. Biochem Biophys Res Commun. 1990 Nov 30;173(1):106–110. doi: 10.1016/s0006-291x(05)81028-9. [DOI] [PubMed] [Google Scholar]
- Hagan R. M., Ireland S. J., Jordan C. C., Beresford I. J., Deal M. J., Ward P. Receptor-selective, peptidase-resistant agonists at neurokinin NK-1 and NK-2 receptors: new tools for investigating neurokinin function. Neuropeptides. 1991 Jun;19(2):127–135. doi: 10.1016/0143-4179(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Hellstrom P. M., Murthy K. S., Grider J. R., Makhlouf G. M. Coexistence of three tachykinin receptors coupled to Ca++ signaling pathways in intestinal muscle cells. J Pharmacol Exp Ther. 1994 Jul;270(1):236–243. [PubMed] [Google Scholar]
- Holzer-Petsche U. Tachykinin receptors in gastrointestinal motility. Regul Pept. 1995 May 4;57(1):19–42. doi: 10.1016/0167-0115(95)00019-8. [DOI] [PubMed] [Google Scholar]
- Holzer P., Maggi C. A. Synergistic role of muscarinic acetylcholine and tachykinin NK-2 receptors in intestinal peristalsis. Naunyn Schmiedebergs Arch Pharmacol. 1994 Feb;349(2):194–201. doi: 10.1007/BF00169837. [DOI] [PubMed] [Google Scholar]
- Kanada A., Hata F., Suthamnatpong N., Maehara T., Ishii T., Takeuchi T., Yagasaki O. Key roles of nitric oxide and cyclic GMP in nonadrenergic and noncholinergic inhibition in rat ileum. Eur J Pharmacol. 1992 Jun 5;216(2):287–292. doi: 10.1016/0014-2999(92)90372-b. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wolf D. Increase by NO synthase inhibitors of acetylcholine release from guinea-pig myenteric plexus. Naunyn Schmiedebergs Arch Pharmacol. 1994 May;349(5):543–545. doi: 10.1007/BF00169145. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Murphy S., Furness J. B., Young H. M., Campbell G. Relationships between nitric oxide synthase, vasoactive intestinal peptide and substance P immunoreactivities in neurons of the amphibian intestine. J Auton Nerv Syst. 1993 Aug-Sep;44(2-3):197–206. doi: 10.1016/0165-1838(93)90032-p. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith I. J., Song Z. M., Costa M., Bredt D. S., Snyder S. H. Ultrastructural localization of nitric oxide synthase immunoreactivity in guinea-pig enteric neurons. Brain Res. 1992 Apr 17;577(2):337–342. doi: 10.1016/0006-8993(92)90294-j. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Patacchini R., Quartara L., Rovero P., Renzetti A. R., Mizrahi J., Giachetti A. Heterogeneity of tachykinin NK-2 receptors in rabbit, guinea-pig and human smooth muscles. Neuropeptides. 1992 Nov;23(3):181–186. doi: 10.1016/0143-4179(92)90120-l. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Giuliani S., Giachetti A. In vivo and in vitro pharmacology of SR 48,968, a non-peptide tachykinin NK2 receptor antagonist. Eur J Pharmacol. 1993 Mar 30;234(1):83–90. doi: 10.1016/0014-2999(93)90709-q. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Meini S., Giuliani S. Nitric oxide is the mediator of tachykinin NK3 receptor-induced relaxation in the circular muscle of the guinea-pig ileum. Eur J Pharmacol. 1993 Aug 10;240(1):45–50. doi: 10.1016/0014-2999(93)90543-q. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Rovero P., Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993 Feb;13(1):23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Zagorodnyuk V., Giuliani S. Tachykinin NK3 receptor mediates NANC hyperpolarization and relaxation via nitric oxide release in the circular muscle of the guinea-pig colon. Regul Pept. 1994 Oct 21;53(3):259–274. doi: 10.1016/0167-0115(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- McLean S., Ganong A., Seymour P. A., Snider R. M., Desai M. C., Rosen T., Bryce D. K., Longo K. P., Reynolds L. S., Robinson G. Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J Pharmacol Exp Ther. 1993 Oct;267(1):472–479. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris S. M., Jr, Billiar T. R. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994 Jun;266(6 Pt 1):E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bueb J. L., Bronner C., Rouot B., Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990 Sep;11(9):358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Mammalian tachykinin receptors. Annu Rev Neurosci. 1991;14:123–136. doi: 10.1146/annurev.ne.14.030191.001011. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993 Apr;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Lördal M., al-Saffar A., Hellström P. M. Intestinal motility responses to neuropeptide gamma in vitro and in vivo in the rat: comparison with neurokinin 1 and neurokinin 2 receptor agonists. Acta Physiol Scand. 1994 Aug;151(4):497–505. doi: 10.1111/j.1748-1716.1994.tb09772.x. [DOI] [PubMed] [Google Scholar]
- Rahman M., Lördal M., al-Saffar A., Hellström P. M. Intestinal motility responses to neuropeptide gamma in vitro and in vivo in the rat: comparison with neurokinin 1 and neurokinin 2 receptor agonists. Acta Physiol Scand. 1994 Aug;151(4):497–505. doi: 10.1111/j.1748-1716.1994.tb09772.x. [DOI] [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Dion S., D'Orléans-Juste P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38(1):1–15. doi: 10.1159/000138512. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Smits G. J., Lefebvre R. A. Tachykinin receptors involved in the contractile effect of the natural tachykinins in the rat gastric fundus. J Auton Pharmacol. 1994 Dec;14(6):383–392. doi: 10.1111/j.1474-8673.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Szurszewski J. H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992 Dec;103(6):1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Mizuno K., Gomi Y. Tachykinin-induced contractions in the circular muscle of guinea pig ileum. Jpn J Pharmacol. 1994 Jul;65(3):233–240. doi: 10.1254/jjp.65.233. [DOI] [PubMed] [Google Scholar]
- Toda N., Baba H., Okamura T. Role of nitric oxide in non-adrenergic, non-cholinergic nerve-mediated relaxation in dog duodenal longitudinal muscle strips. Jpn J Pharmacol. 1990 Jun;53(2):281–284. doi: 10.1254/jjp.53.281. [DOI] [PubMed] [Google Scholar]
- Wang L., Stanisz A. M., Wershil B. K., Galli S. J., Perdue M. H. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am J Physiol. 1995 Jul;269(1 Pt 1):G85–G92. doi: 10.1152/ajpgi.1995.269.1.G85. [DOI] [PubMed] [Google Scholar]
- Xue C., Pollock J., Schmidt H. H., Ward S. M., Sanders K. M. Expression of nitric oxide synthase immunoreactivity by interstitial cells of the canine proximal colon. J Auton Nerv Syst. 1994 Sep;49(1):1–14. doi: 10.1016/0165-1838(94)90015-9. [DOI] [PubMed] [Google Scholar]