Abstract
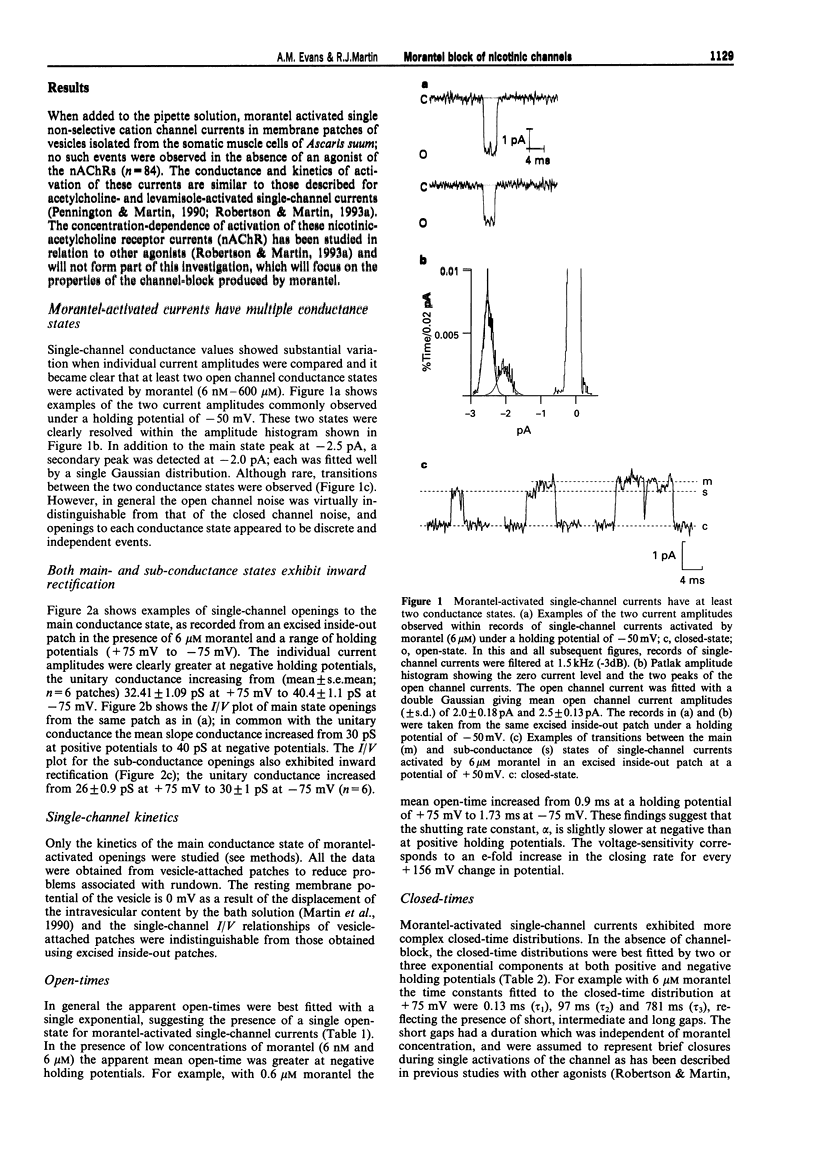
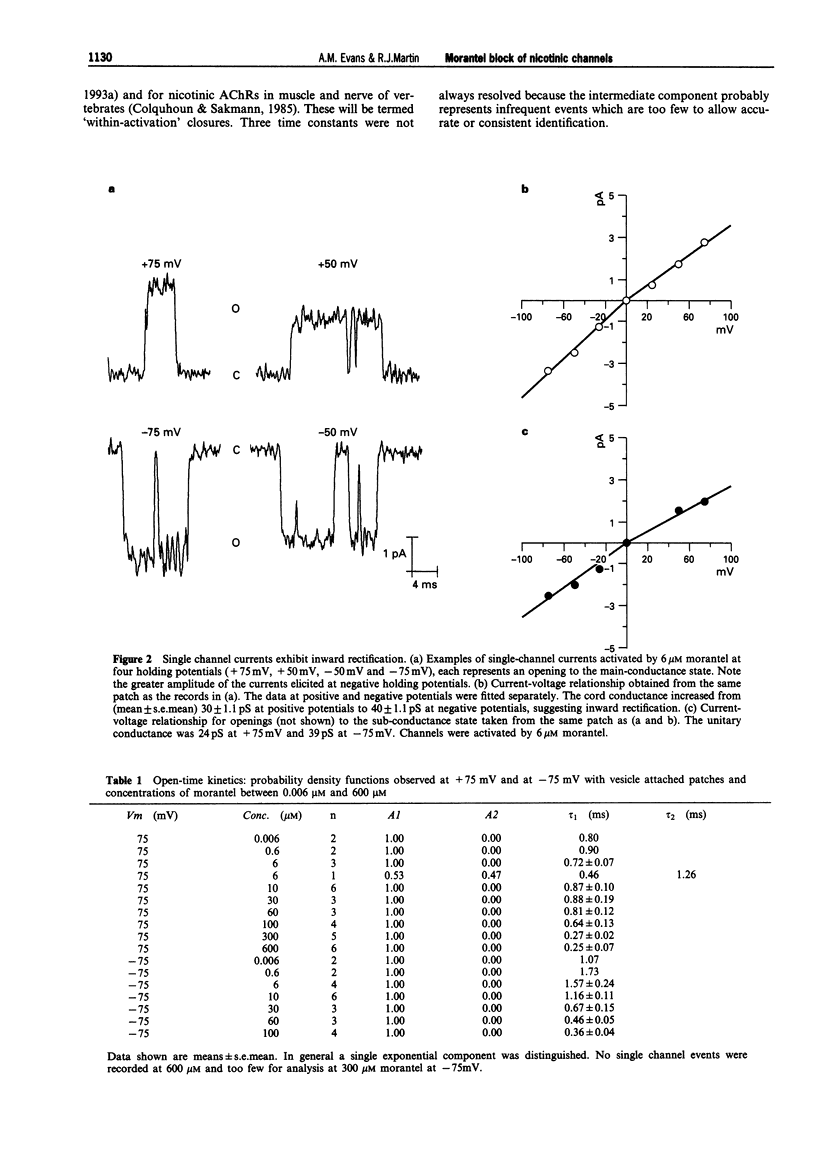
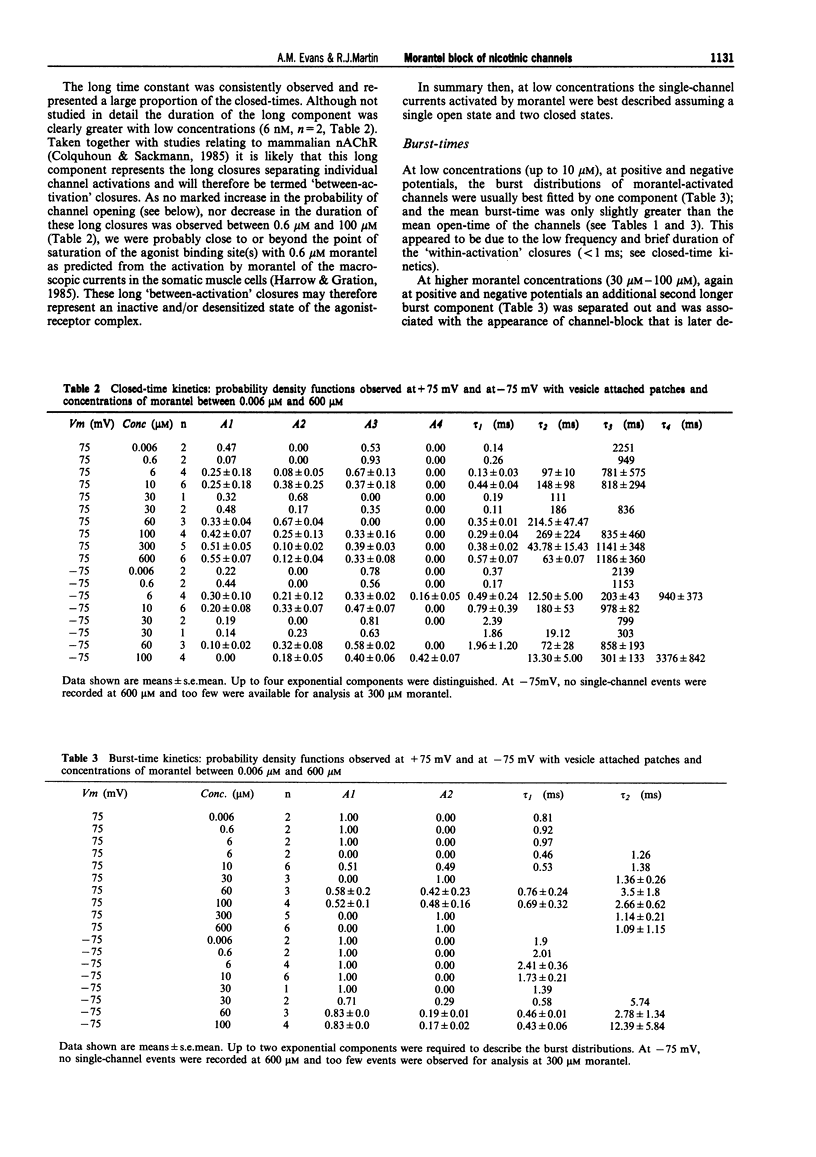
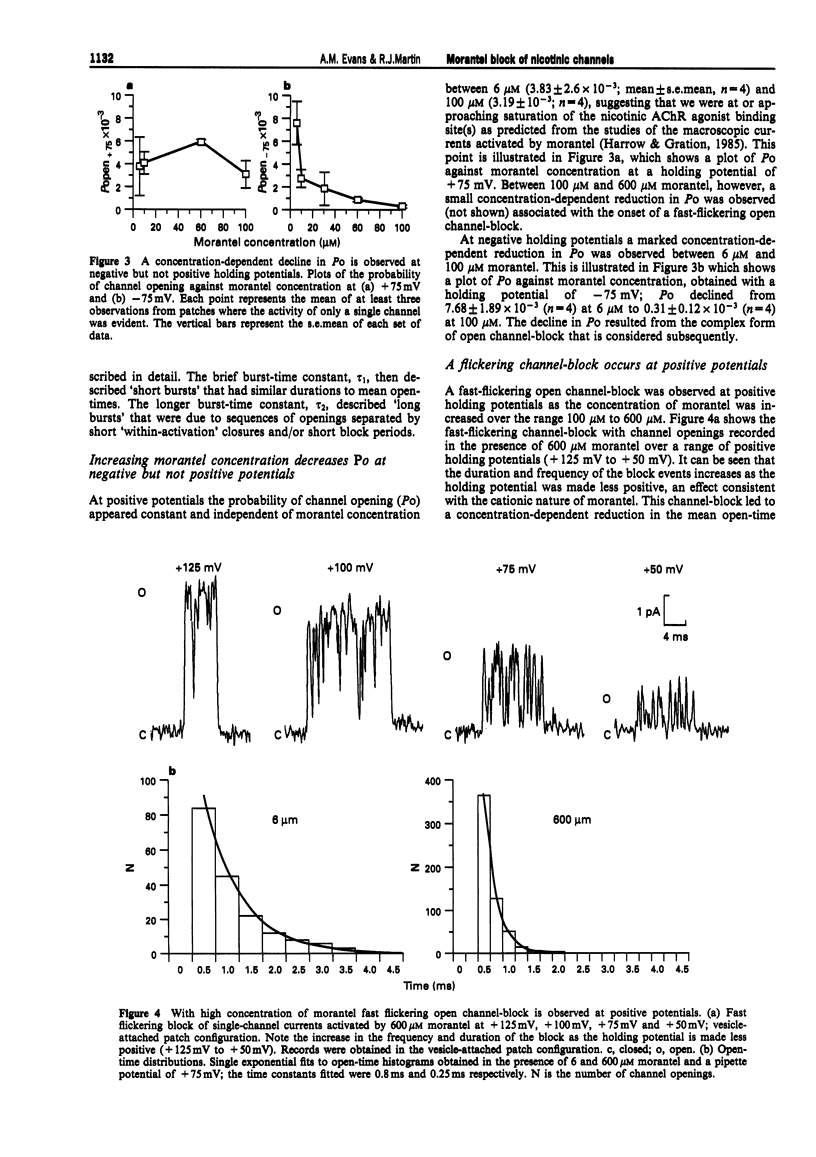
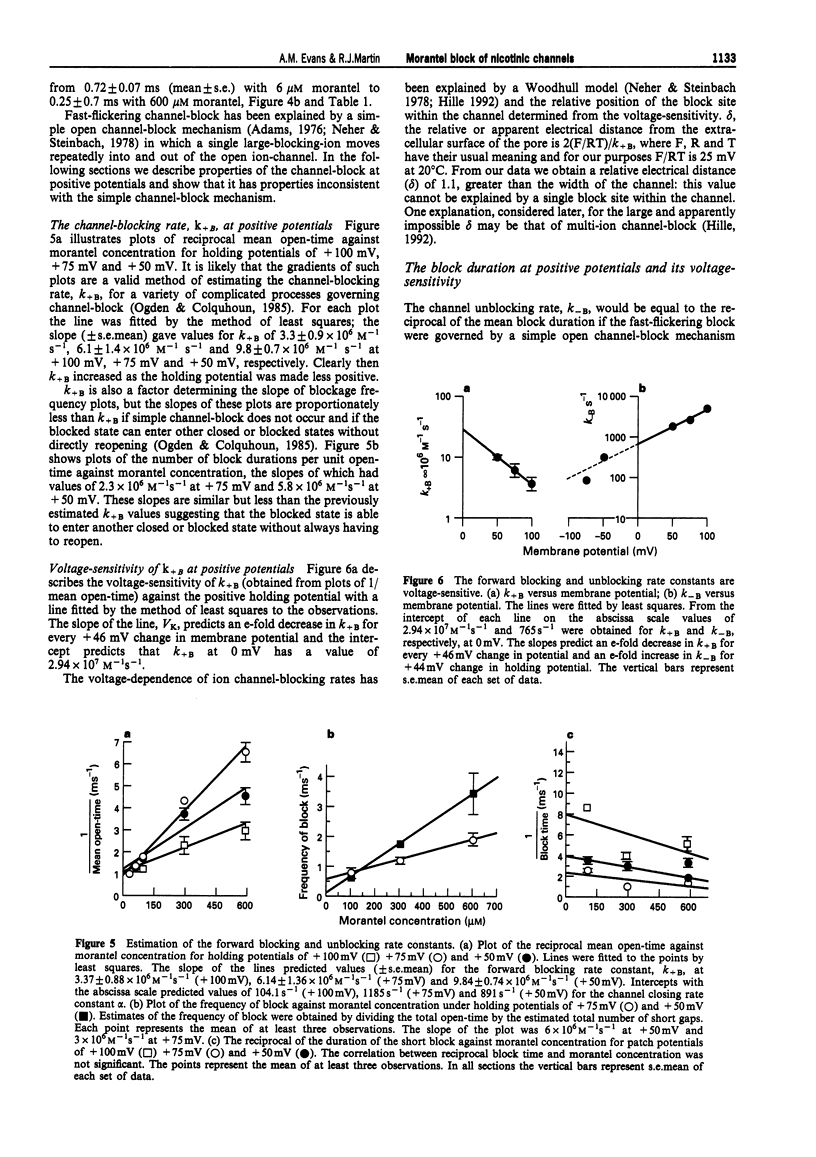
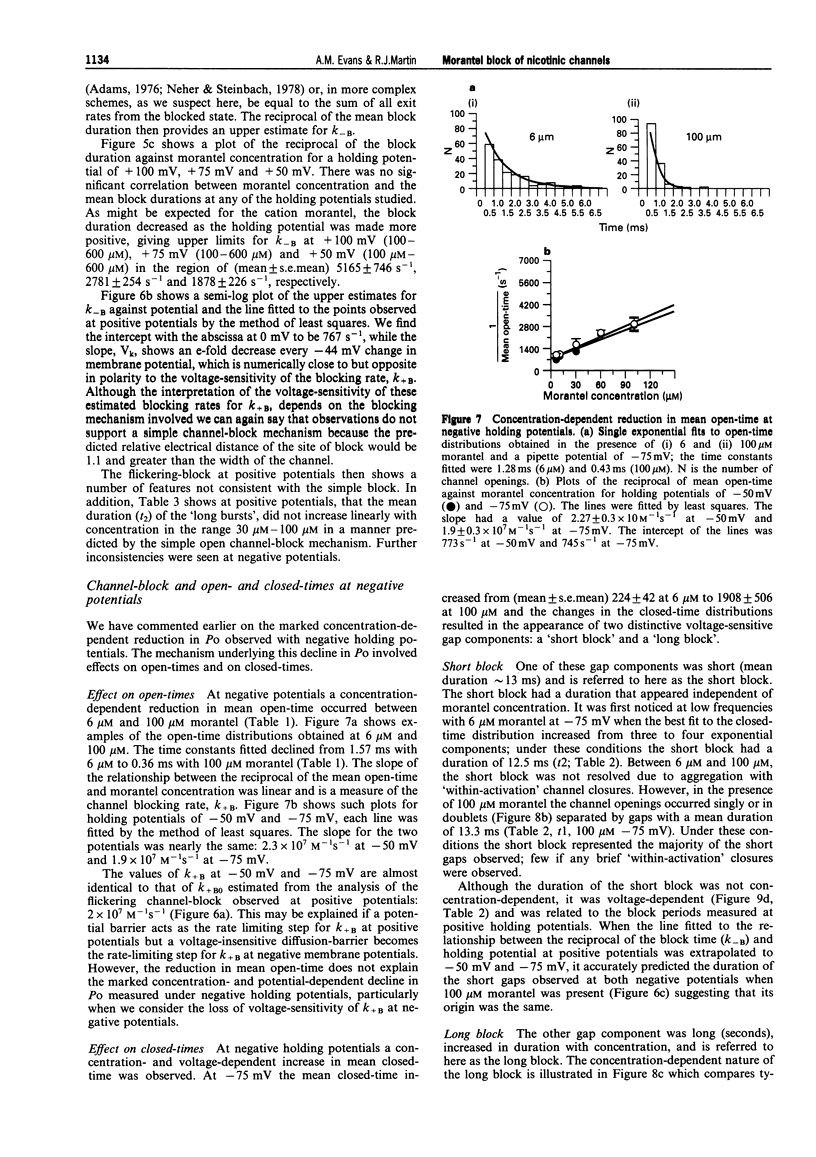
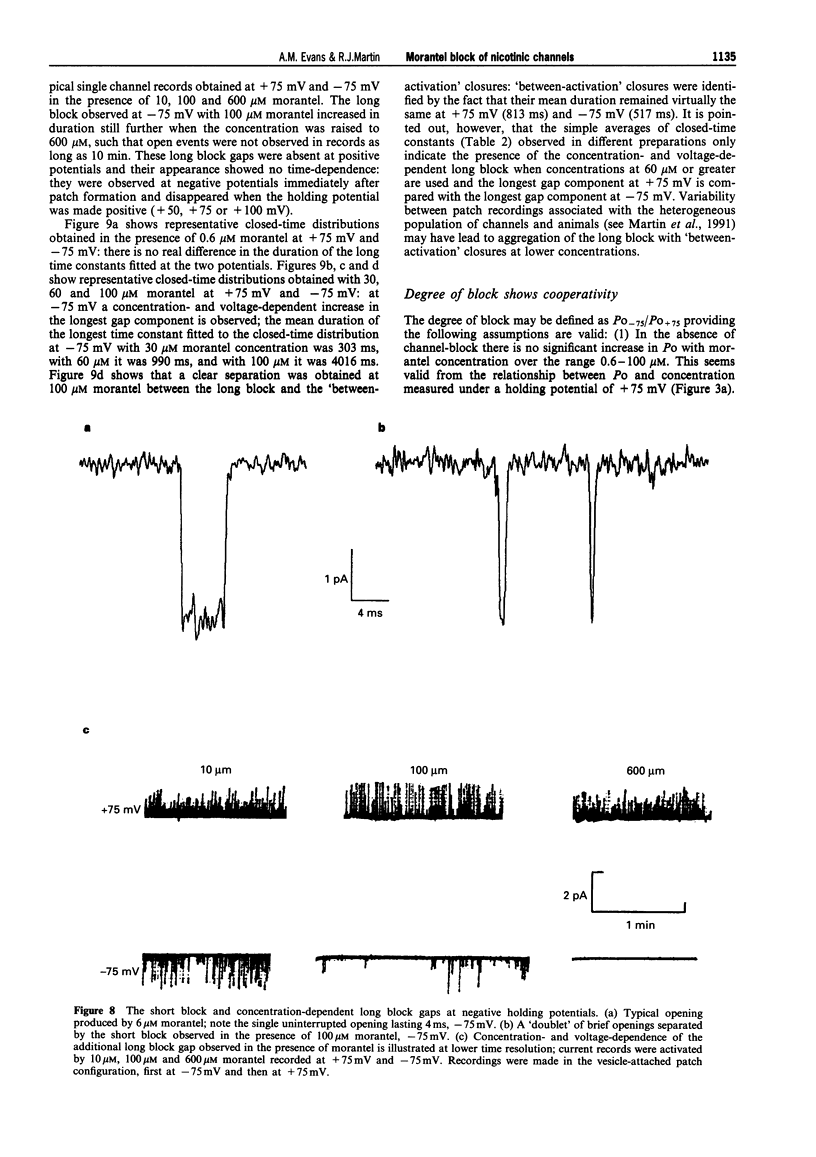
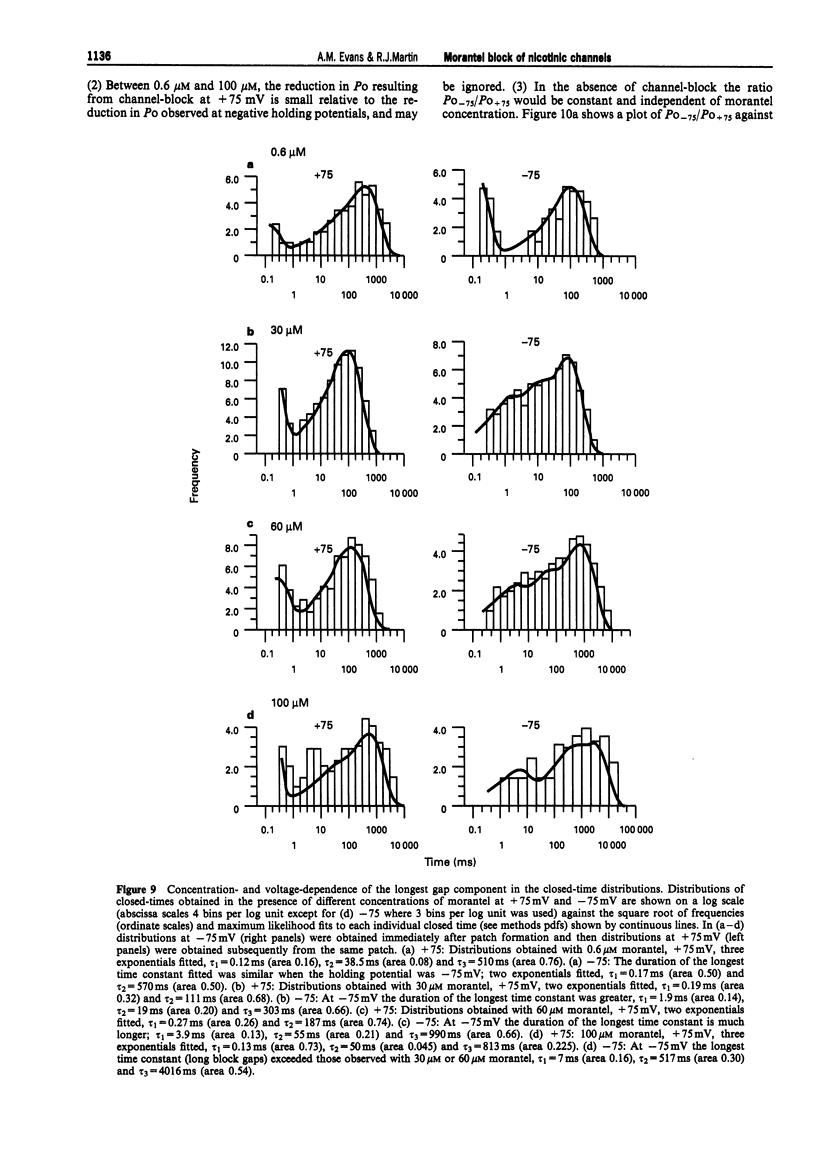
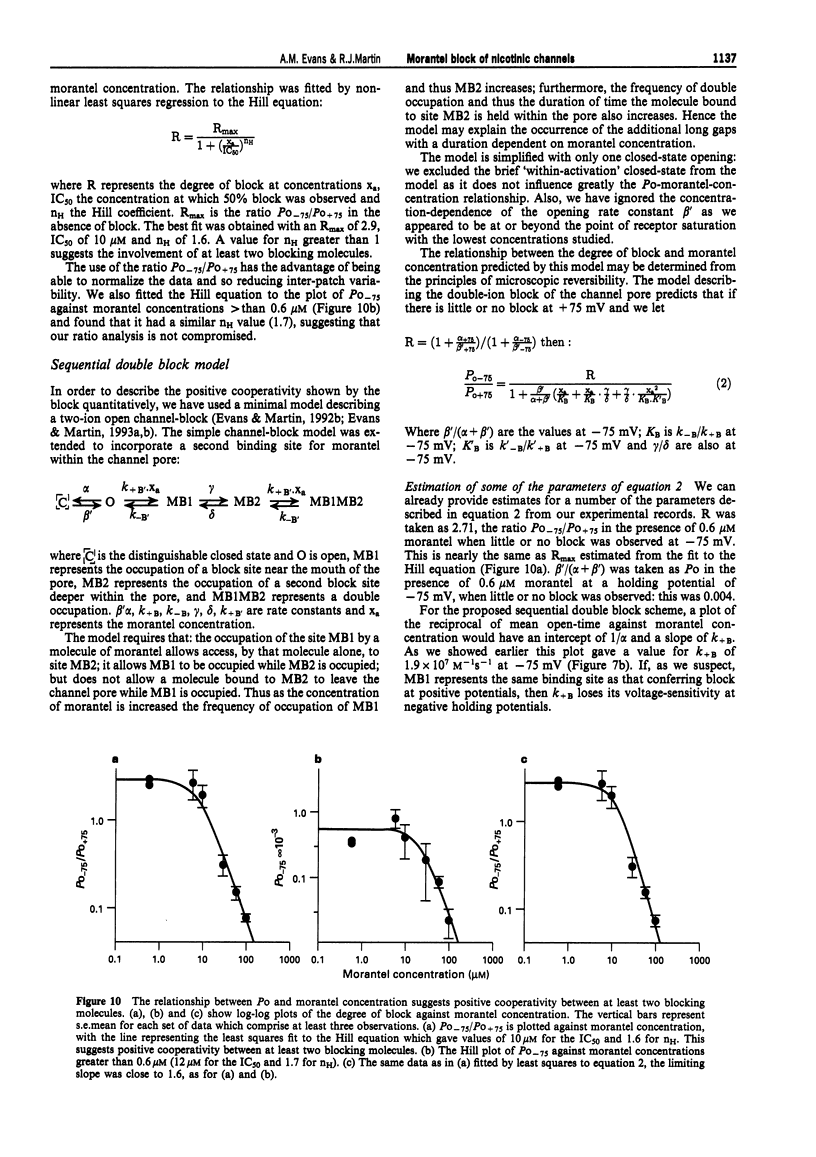
1. We have investigated activation and block, by the tetrahydropyrimidine anthelmintic, morantel, of nicotinic-acetylcholine receptor (AChR) currents in membrane vesicles isolated from somatic muscle cells of the nematode parasite Ascaris suum. Standard single-channel recording techniques were employed. Morantel in the pipette (6 nM to 600 microM), activated single nicotinic AChR currents. 2. Kinetic properties of the main-conductance state of morantel-activated currents were investigated in detail throughout the concentration range, 0.6 microM to 600 microM. Open-time distributions were best fitted by a single exponential. Mean open-times were slightly voltage-dependent, increasing from 0.9 ms at +75 mV to 1.74 ms at -75 mV in the presence of 0.6 microM morantel. At low concentrations, closed-time distributions were best fitted by the sum of two or three exponential components. 3. As the concentration of morantel was increased (100-600 microM), fast-flickering open channel-block was observed at positive potentials, even though morantel, a cation, was only present at the extracellular surface of the membrane. The block rate was dependent on morantel concentration and both block rate and duration of block increased as the potential became less positive. A simple channel-block mechanism did not explain properties of this block. 4. At negative potentials, as the morantel concentration increased, a complex block was observed. With increases in morantel concentration two additional gap components appeared in closed-time distributions: one was short with a duration (approximately 13 ms) independent of morantel concentration; the other was long with a duration that increased with morantel concentration (up to many minutes). In combination, these two components produced a marked reduction in probability of channel opening (Po) with increasing morantel concentration. The relationship between the degrees of block and morantel concentration had a Hill coefficient of 1.6, suggesting the involvement of at least two blocking molecules. The data were analysed by use of a simple sequential double block kinetic model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, French R. J. Blocking of the squid axon potassium channel by external caesium ions. J Physiol. 1978 Mar;276:13–25. doi: 10.1113/jphysiol.1978.sp012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN E., MOYLE V. A contribution to the physiology and pharmacology of Ascaris lumbricoides from the pig. Br J Pharmacol Chemother. 1949 Jun;4(2):145–152. doi: 10.1111/j.1476-5381.1949.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Spruce A. E., Standen N. B., Stanfield P. R. Multiple blocking mechanisms of ATP-sensitive potassium channels of frog skeletal muscle by tetraethylammonium ions. J Physiol. 1989 Jun;413:31–48. doi: 10.1113/jphysiol.1989.sp017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola M., Kao P. N., Karlin A. Mapping the alpha-subunit site photolabeled by the noncompetitive inhibitor [3H]quinacrine azide in the active state of the nicotinic acetylcholine receptor. J Biol Chem. 1990 Jul 5;265(19):11017–11029. [PubMed] [Google Scholar]
- Fredkin D. R., Rice J. A. Maximum likelihood estimation and identification directly from single-channel recordings. Proc Biol Sci. 1992 Aug 22;249(1325):125–132. doi: 10.1098/rspb.1992.0094. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Gingrich K. J., Beardsley D., Yue D. T. Ultra-deep blockade of Na+ channels by a quaternary ammonium ion: catalysis by a transition-intermediate state? J Physiol. 1993 Nov;471:319–341. doi: 10.1113/jphysiol.1993.sp019903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Lester H. A. The permeation pathway of neurotransmitter-gated ion channels. Annu Rev Biophys Biomol Struct. 1992;21:267–292. doi: 10.1146/annurev.bb.21.060192.001411. [DOI] [PubMed] [Google Scholar]
- Maconochie D. J., Steinbach J. H. Block by acetylcholine of mouse muscle nicotinic receptors, stably expressed in fibroblasts. J Gen Physiol. 1995 Jul;106(1):113–147. doi: 10.1085/jgp.106.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. Further studies of the effect of calcium on the time course of action of carbamylcholine at the neuromuscular junction. J Gen Physiol. 1970 Sep;56(3):407–419. doi: 10.1085/jgp.56.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982 Oct;77(2):255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Kusel J. R., Pennington A. J. Surface properties of membrane vesicles prepared from muscle cells of Ascaris suum. J Parasitol. 1990 Jun;76(3):340–348. [PubMed] [Google Scholar]
- Martin R. J., Pennington A. J., Duittoz A. H., Robertson S., Kusel J. R. The physiology and pharmacology of neuromuscular transmission in the nematode parasite, Ascaris suum. Parasitology. 1991;102 (Suppl):S41–S58. doi: 10.1017/s0031182000073285. [DOI] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Natoff I. L. The pharmacology of the cholinoceptor in muscle preparations of Ascaris lumbricoides var. suum. Br J Pharmacol. 1969 Sep;37(1):251–257. doi: 10.1111/j.1476-5381.1969.tb09542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington A. J., Martin R. J. A patch-clamp study of acetylcholine-activated ion channels in Ascaris suum muscle. J Exp Biol. 1990 Nov;154:201–221. doi: 10.1242/jeb.154.1.201. [DOI] [PubMed] [Google Scholar]
- Robertson S. J., Martin R. J. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br J Pharmacol. 1993 Jan;108(1):170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhkova E. K., Malyutina T. A., Shishov B. A. Pharmacological characteristics of cholinoreception in somatic muscles of the nematode, Ascaris suum. Gen Pharmacol. 1980;11(1):141–146. doi: 10.1016/0306-3623(80)90023-3. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Zhorov B. S., Brovtsyna N. B., Gmiro V. E., Lukomskaya NYa, Serdyuk S. E., Potapyeva N. N., Magazanik L. G., Kurenniy D. E., Skok V. I. Dimensions of the ion channel in neuronal nicotinic acetylcholine receptor as estimated from analysis of conformation-activity relationships of open-channel blocking drugs. J Membr Biol. 1991 Apr;121(2):119–132. doi: 10.1007/BF01870527. [DOI] [PubMed] [Google Scholar]



