Abstract
1. The aim of this work was to investigate the effect of lipopolysaccharide (LPS) treatment on the relationship between the cytosolic Ca2+ ion concentration ([Ca2+]i) and contraction in rat resistance arteries, and the involvement of the L-arginine-nitric oxide (NO)-guanosine 3'-5' cyclic monophosphate (cyclic GMP) pathway in these effects. 2. [Ca2+]i and tension were simultaneously recorded in small mesenteric arteries removed from rats 4 h after intraperitoneal injection of E. coli LPS (30 mg kg-1) or solvent. Cyclic GMP was assayed in vessels submitted to identical treatments. 3. Basal [Ca2+]i was higher in vessels from LPS-treated rats compared to controls. LPS did not modify the concentration-contraction curve of noradrenaline. However, the increase in basal [Ca2+]i produced by LPS resulted in a shift of the noradrenaline [Ca2+]i-contraction curve to higher [Ca2+]i concentrations. 4. L-Arginine (300 microM) relaxed noradrenaline (10 microM) pre-contracted arteries from LPS-treated but not from control rats. This effect of L-arginine was reversed by two inhibitors of NO synthase: N omega-nitro-L-arginine-methyl-ester (L-NAME, 1 mM) and S-methyl-isothiourea (SMT, 0.1 mM). Both the relaxing effect of L-arginine and its reversal by L-NAME or SMT occurred without any change in [Ca2+]i. 5. LPS treatment did not modify the cyclic GMP content of the small mesenteric arteries. In arteries removed from LPS-treated rats but not from controls, addition of L-arginine (300 microM) was associated with a significant increase in cyclic GMP content, an effect which was prevented by both L-NAME (1 mM) and SMT (0.1 mM). 6. L-NAME (1 mM) produced a greater reduction in cyclic GMP content than SMT (0.1 mM) in control vessels exposed to L-arginine (300 microM). Under the same conditions, SMT produced a larger decrease in cyclic GMP level than L-NAME in arteries taken from LPS-treated rats, consistent with selective inhibition by SMT of the inducible NO-synthase after LPS. 7. These results show that LPS produced two effects in small mesenteric arteries: (i) alterations in Ca2+ handling and a decreased sensitivity of myofilaments to Ca2+, (ii) induction of NO-synthase activity resulting in exogenous L-arginine-dependent production of NO and cyclic GMP accumulation. Both effects are likely to be involved in the hyporeactivity induced by LPS in resistance arteries.
Full text
PDF
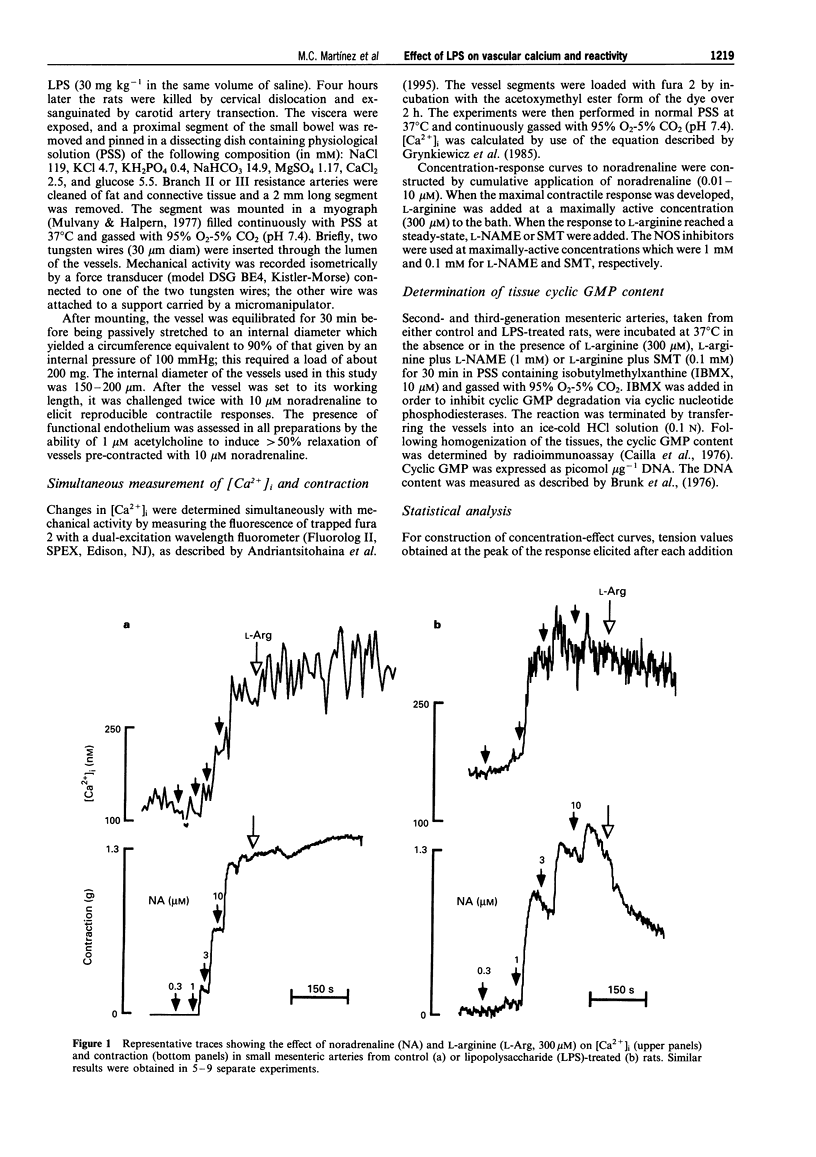
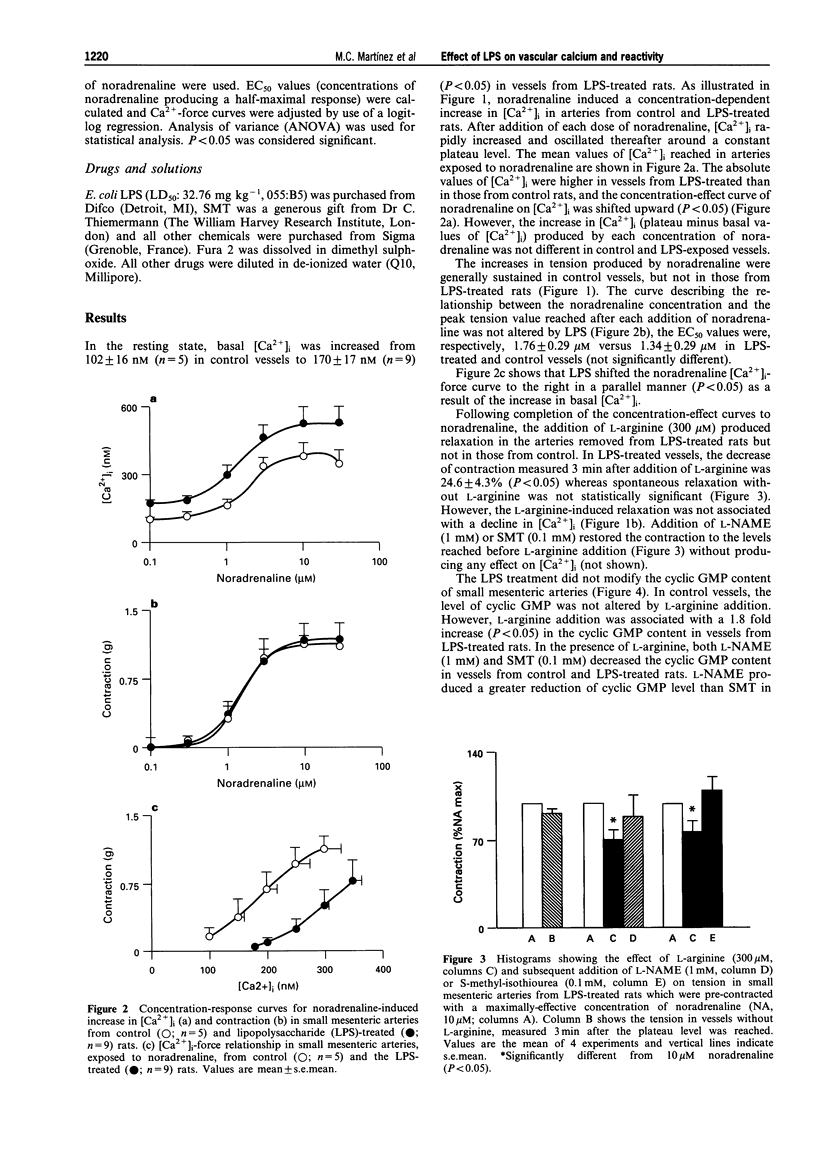
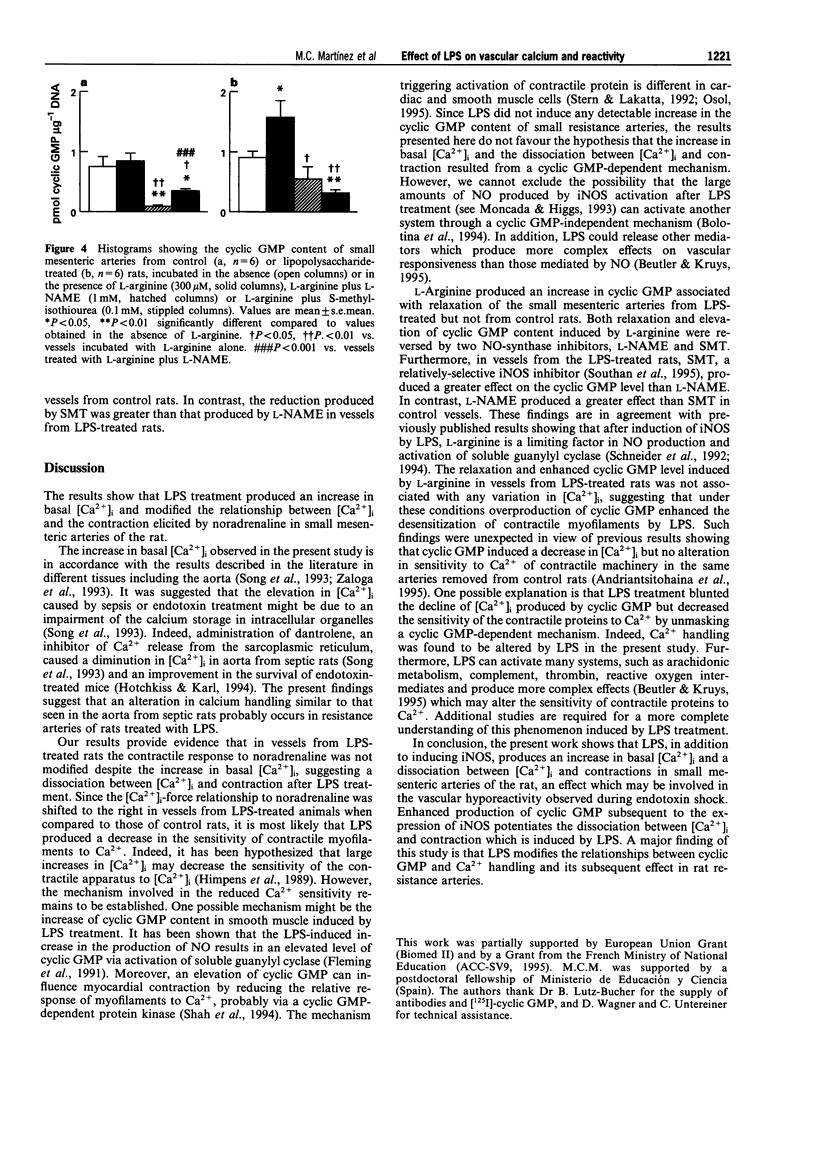

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriantsitohaina R., Lagaud G. J., Andre A., Muller B., Stoclet J. C. Effects of cGMP on calcium handling in ATP-stimulated rat resistance arteries. Am J Physiol. 1995 Mar;268(3 Pt 2):H1223–H1231. doi: 10.1152/ajpheart.1995.268.3.H1223. [DOI] [PubMed] [Google Scholar]
- Beutler B., Kruys V. Lipopolysaccharide signal transduction, regulation of tumor necrosis factor biosynthesis, and signaling by tumor necrosis factor itself. J Cardiovasc Pharmacol. 1995;25 (Suppl 2):S1–S8. doi: 10.1097/00005344-199500252-00002. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Vannier C. J., Delaage M. A. Guanosine 3', 5'-cyclicmonophosphate assay at 10(-15)-mole level. Anal Biochem. 1976 Jan;70(1):195–202. doi: 10.1016/s0378-5173(83)90100-x. [DOI] [PubMed] [Google Scholar]
- Fleming I., Julou-Schaeffer G., Gray G. A., Parratt J. R., Stoclet J. C. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br J Pharmacol. 1991 May;103(1):1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989 Jun;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. S., Karl I. E. Dantrolene ameliorates the metabolic hallmarks of sepsis in rats and improves survival in a mouse model of endotoxemia. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3039–3043. doi: 10.1073/pnas.91.8.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Kohlhaas K. L., Sorrentino R., Warner T. D., Murad F., Vane J. R. Induction by endotoxin of nitric oxide synthase in the rat mesentery: lack of effect on action of vasoconstrictors. Br J Pharmacol. 1993 May;109(1):265–270. doi: 10.1111/j.1476-5381.1993.tb13563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995 Sep-Oct;32(5):275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- Portolés M. T., Ainaga M. J., Municio A. M., Pagani R. Intracellular calcium and pH alterations induced by Escherichia coli endotoxin in rat hepatocytes. Biochim Biophys Acta. 1991 Mar 19;1092(1):1–6. doi: 10.1016/0167-4889(91)90170-3. [DOI] [PubMed] [Google Scholar]
- Schneider F., Schott C., Stoclet J. C., Julou-Schaeffer G. L-arginine induces relaxation of small mesenteric arteries from endotoxin-treated rats. Eur J Pharmacol. 1992 Feb 11;211(2):269–272. doi: 10.1016/0014-2999(92)90539-g. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Spurgeon H. A., Sollott S. J., Talo A., Lakatta E. G. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res. 1994 May;74(5):970–978. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- Song S. K., Karl I. E., Ackerman J. J., Hotchkiss R. S. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proc Natl Acad Sci U S A. 1993 May 1;90(9):3933–3937. doi: 10.1073/pnas.90.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan G. J., Szabó C., Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995 Jan;114(2):510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Lakatta E. G. Excitation-contraction coupling in the heart: the state of the question. FASEB J. 1992 Sep;6(12):3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- Zaloga G. P., Washburn D., Black K. W., Prielipp R. Human sepsis increases lymphocyte intracellular calcium. Crit Care Med. 1993 Feb;21(2):196–202. doi: 10.1097/00003246-199302000-00009. [DOI] [PubMed] [Google Scholar]