Abstract
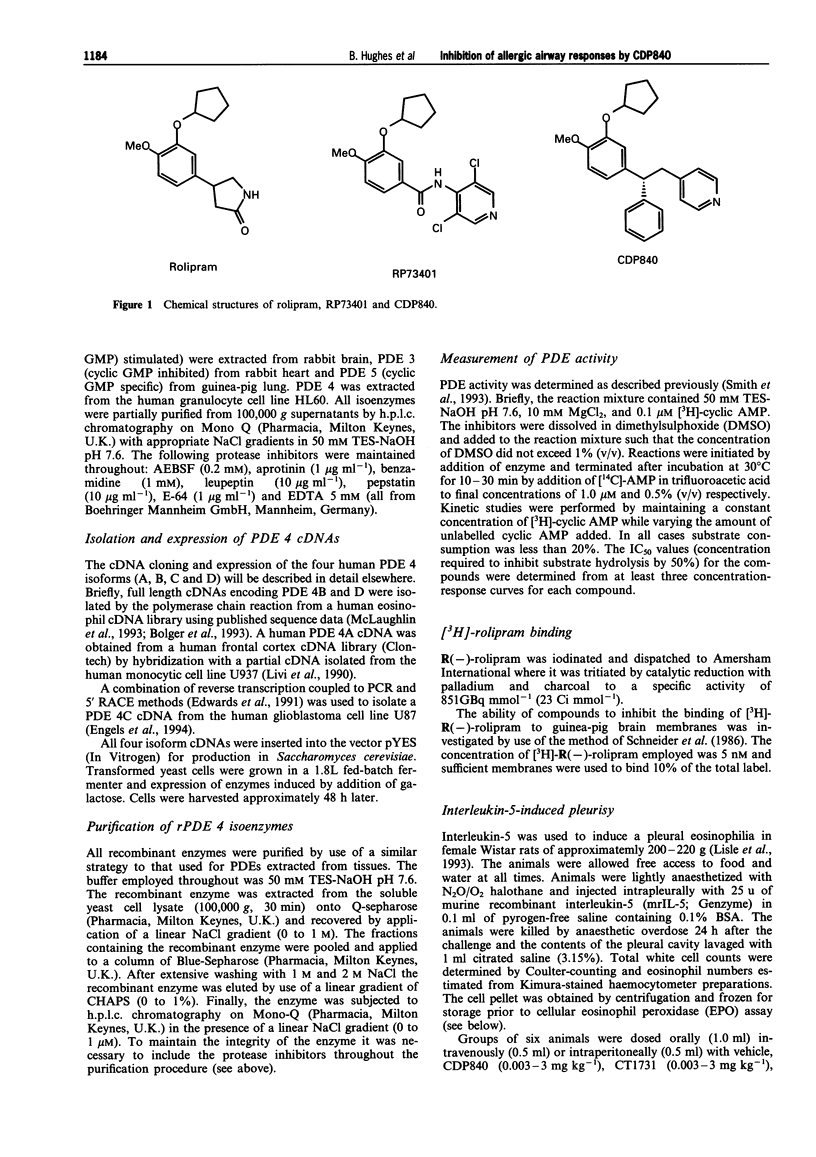
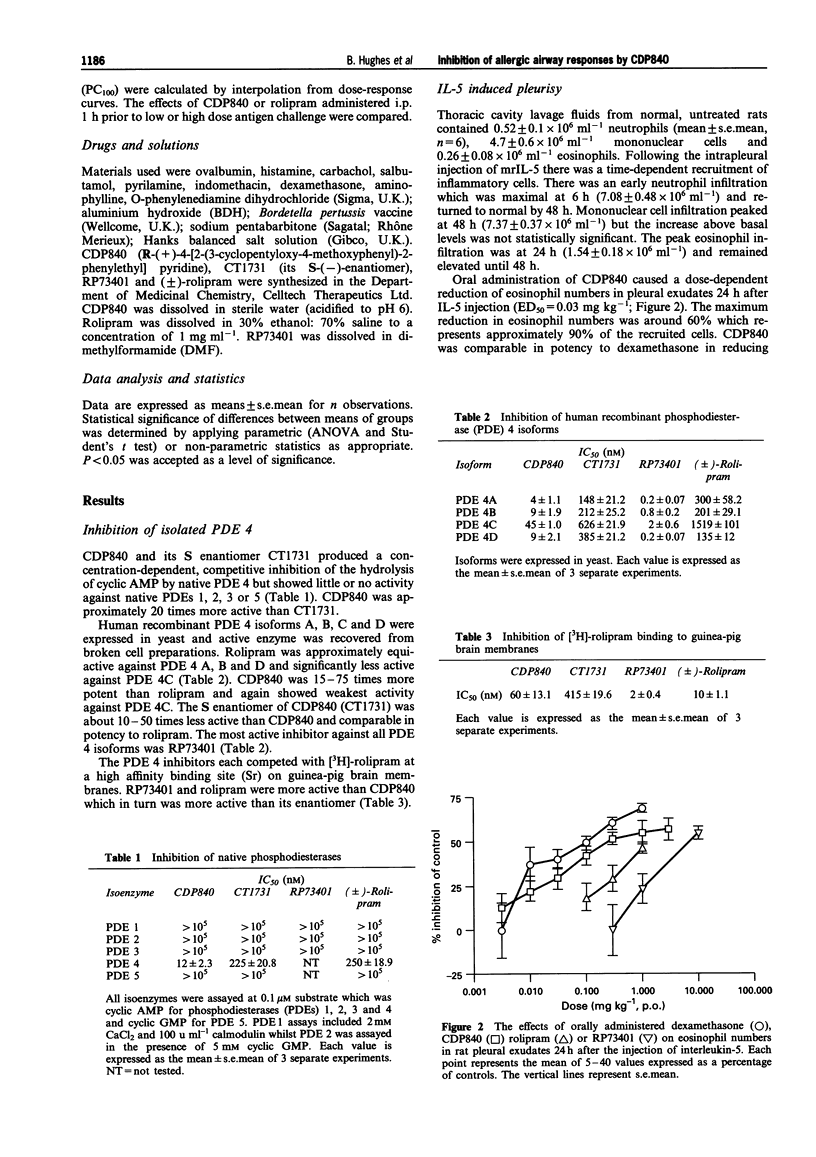
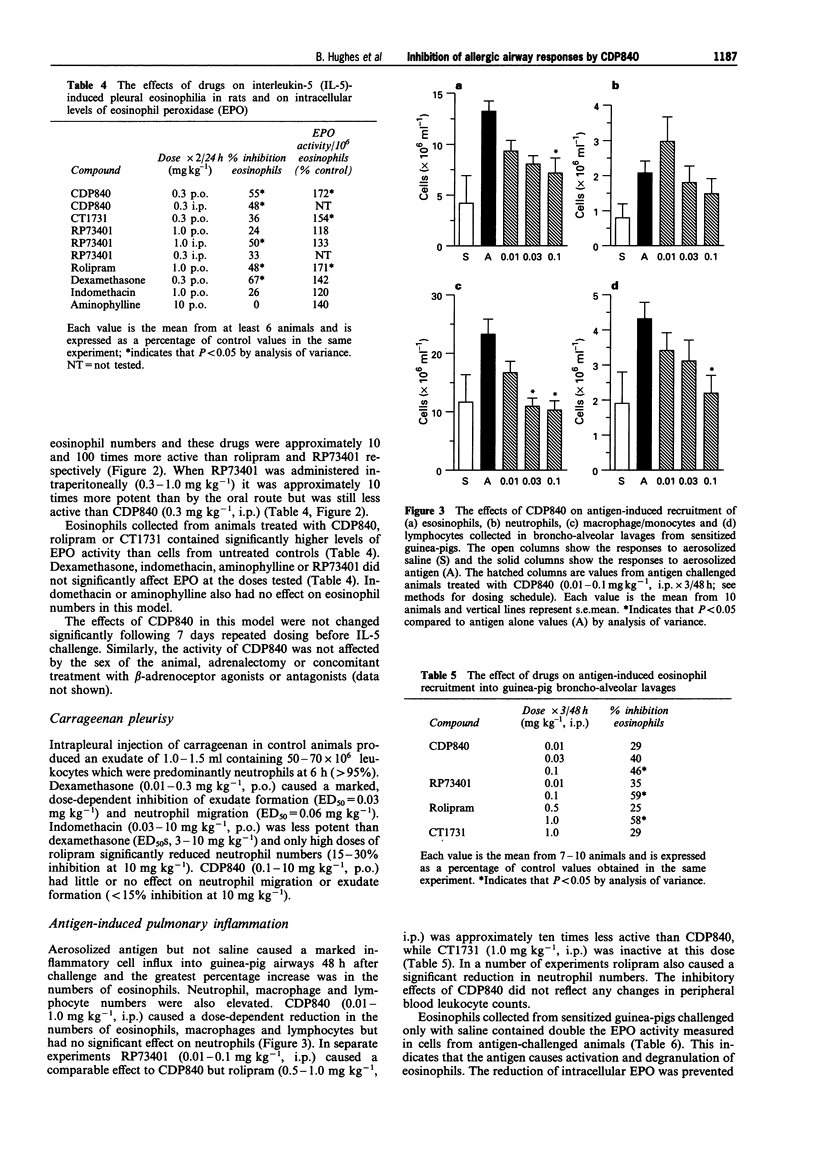
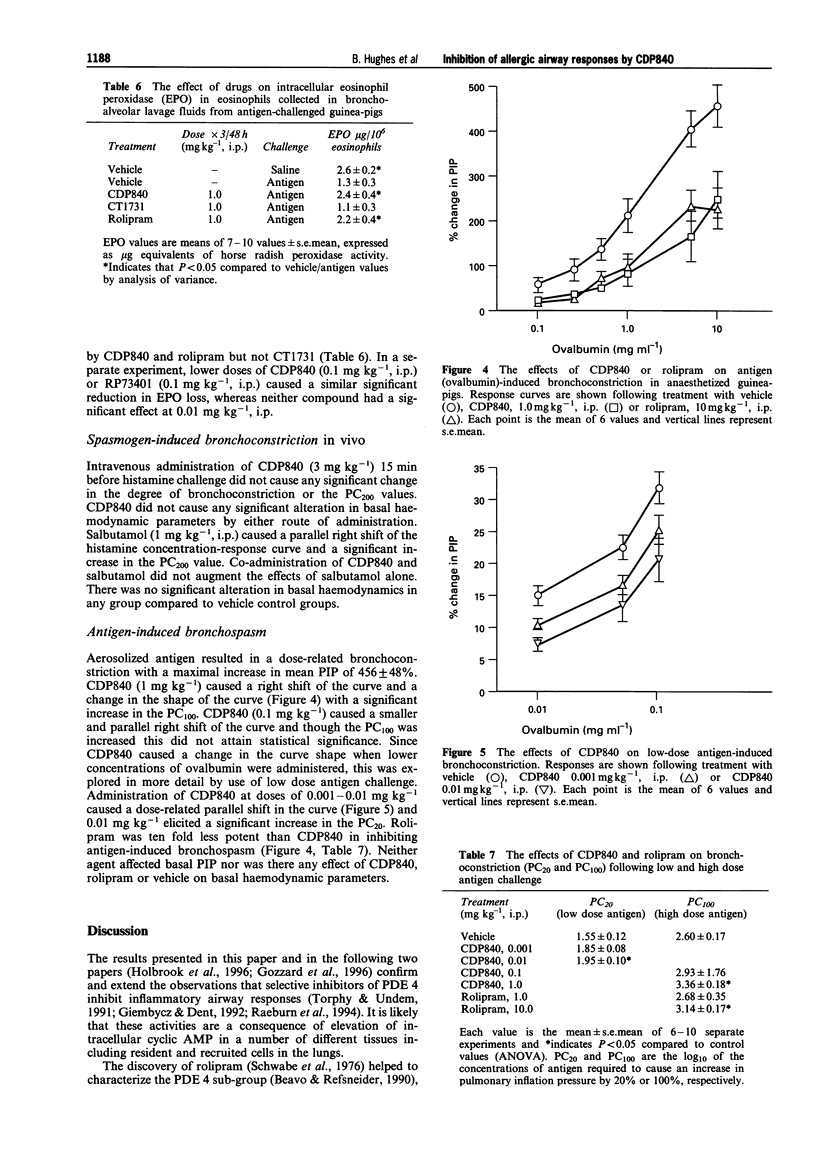
1. The novel tri-aryl ethane CDP840, is a potent and selective inhibitor of cyclic AMP phosphodiesterase type 4 (PDE 4) extracted from tissues or recombinant PDE 4 isoforms expressed in yeast (IC50S: 4-45 nM). CDP840 is stereo-selective since its S enantiomer (CT 1731) is 10-50 times less active against all forms of PDE 4 tested while both enantiomers are inactive (IC50S: > 100 microM) against PDE types 1, 2, 3 and 5. 2. Oral administration of CDP840 caused a dose-dependent reduction of interleukin-5 (IL-5)-induced pleural eosinophilia in rats (ED50 = 0.03 mg kg-1). The eosinophils in pleural exudates from CDP840-treated animals contained higher levels of eosinophil peroxidase (EPO) than cells from control animals, suggesting a stabilizing effect on eosinophil degranulation. CDP840 was approximately equi-active with the steroid dexamethasone in this model and was 10-100 times more potent than the known PDE 4-selective inhibitors rolipram and RP73401. The activity of CDP840 was not influenced by adrenalectomy, beta-sympathomimetics or beta-sympatholytics. 3. Antigen-induced pulmonary eosinophilia in sensitized guinea-pigs was reduced dose-dependently by CDP840 (0.01-1 mg kg-1, i.p.) and intracellular EPO levels were significantly higher. CDP840 was more potent in these activities than CT1731 or rolipram and comparable in potency to RP73401. 4. Rolipram or CDP840 were less active than dexamethasone in preventing neutrophil accumulation, or exudate formation in carrageenan-induced pleurisy in rats and thus do not exhibit general anti-inflammatory activity. 5. In sensitized guinea-pigs, aerosols of the antigen ovalbumin caused a dose-dependent bronchoconstriction demonstrated by an increase in pulmonary inflation pressure. Administration of CDP840 (0.001-1.0 mg kg-1, i.p.), 1 h before antigen challenge, resulted in dose-dependent reduction in response to antigen. This activity was not due to bronchodilatation since higher doses of CDP840 (3 mg kg-1) did not significantly change the bronchoconstrictor response to histamine. Rolipram was approximately 10 times less active than CDP840 in preventing antigen-induced bronchoconstriction. 6. These results confirm the observations that selective PDE 4 inhibitors reduce antigen-induced bronchoconstriction and pulmonary eosinophilic inflammation. CDP840 is more potent than rolipram in inhibiting native or recombinant PDE 4. Unlike the recently described potent PDE 4 inhibitor RP73401, CDP840 is more active than rolipram in the rat IL-5 model following oral administration. The novel series of tri-aryl ethanes, of which CDP840 is the lead compound, could be the basis of an orally active prophylactic treatment for human asthma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bolger G., Michaeli T., Martins T., St John T., Steiner B., Rodgers L., Riggs M., Wigler M., Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993 Oct;13(10):6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbo M., Horowitz E. M., McKenzie-White J., Kagey-Sobotka A., Lichtenstein L. M. Pharmacologic control of histamine release from human basophils induced by platelet-activating factor. Int Arch Allergy Immunol. 1993;102(4):383–390. doi: 10.1159/000236587. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- Edwards J. B., Delort J., Mallet J. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5' ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 1991 Oct 11;19(19):5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels P., Sullivan M., Müller T., Lübbert H. Molecular cloning and functional expression in yeast of a human cAMP-specific phosphodiesterase subtype (PDE IV-C). FEBS Lett. 1995 Jan 30;358(3):305–310. doi: 10.1016/0014-5793(94)01460-i. [DOI] [PubMed] [Google Scholar]
- Fernandes L. B., Ellis J. L., Undem B. J. Potentiation of nonadrenergic noncholinergic relaxation of human isolated bronchus by selective inhibitors of phosphodiesterase isozymes. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 1):1384–1390. doi: 10.1164/ajrccm.150.5.7952568. [DOI] [PubMed] [Google Scholar]
- Giembycz M. A., Dent G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin Exp Allergy. 1992 Mar;22(3):337–344. doi: 10.1111/j.1365-2222.1992.tb03095.x. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Gozzard N., el-Hashim A., Herd C. M., Blake S. M., Holbrook M., Hughes B., Higgs G. A., Page C. P. Effect of the glucocorticosteroid budesonide and a novel phosphodiesterase type 4 inhibitor CDP840 on antigen-induced airway responses in neonatally immunised rabbits. Br J Pharmacol. 1996 Jul;118(5):1201–1208. doi: 10.1111/j.1476-5381.1996.tb15524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook M., Gozzard N., James T., Higgs G., Hughes B. Inhibition of bronchospasm and ozone-induced airway hyperresponsiveness in the guinea-pig by CDP840, a novel phosphodiesterase type 4 inhibitor. Br J Pharmacol. 1996 Jul;118(5):1192–1200. doi: 10.1111/j.1476-5381.1996.tb15523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell R. E., Sickels B. D., Woeppel S. L. Pulmonary antiallergic and bronchodilator effects of isozyme-selective phosphodiesterase inhibitors in guinea pigs. J Pharmacol Exp Ther. 1993 Feb;264(2):609–615. [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. M., Cieslinski L. B., Burman M., Torphy T. J., Livi G. P. A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNA. J Biol Chem. 1993 Mar 25;268(9):6470–6476. [PubMed] [Google Scholar]
- Pober J. S., Slowik M. R., De Luca L. G., Ritchie A. J. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993 Jun 1;150(11):5114–5123. [PubMed] [Google Scholar]
- Qian Y., Girard V., Martin C. A., Molimard M., Advenier C. Rolipram, but not siguazodan or zaprinast, inhibits the excitatory noncholinergic neurotransmission in guinea-pig bronchi. Eur Respir J. 1994 Feb;7(2):306–310. doi: 10.1183/09031936.94.07020306. [DOI] [PubMed] [Google Scholar]
- Raeburn D., Underwood S. L., Lewis S. A., Woodman V. R., Battram C. H., Tomkinson A., Sharma S., Jordan R., Souness J. E., Webber S. E. Anti-inflammatory and bronchodilator properties of RP 73401, a novel and selective phosphodiesterase type IV inhibitor. Br J Pharmacol. 1994 Dec;113(4):1423–1431. doi: 10.1111/j.1476-5381.1994.tb17156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjar S., Aoki S., Kristersson A., Smith D., Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990 Apr;99(4):679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. H., Schmiechen R., Brezinski M., Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986 Aug 7;127(1-2):105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Miyake M., Ohga Y., Daly J. W. 4-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3',5'-monophosphate phosphodiesterases in homogenates and tissue slices from rat brain. Mol Pharmacol. 1976 Nov;12(6):900–910. [PubMed] [Google Scholar]
- Smith B. J., Wales M. R., Jappy J. W., Perry M. J. A phosphodiesterase assay using alumina microcolumns. Anal Biochem. 1993 Oct;214(1):355–357. doi: 10.1006/abio.1993.1508. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Barker J. N., Morris R. W., MacDonald D. M., Lee T. H. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993 Sep 15;151(6):3274–3282. [PubMed] [Google Scholar]
- Souness J. E., Carter C. M., Diocee B. K., Hassall G. A., Wood L. J., Turner N. C. Characterization of guinea-pig eosinophil phosphodiesterase activity. Assessment of its involvement in regulating superoxide generation. Biochem Pharmacol. 1991 Jul 25;42(4):937–945. doi: 10.1016/0006-2952(91)90056-b. [DOI] [PubMed] [Google Scholar]
- Souness J. E., Maslen C., Webber S., Foster M., Raeburn D., Palfreyman M. N., Ashton M. J., Karlsson J. A. Suppression of eosinophil function by RP 73401, a potent and selective inhibitor of cyclic AMP-specific phosphodiesterase: comparison with rolipram. Br J Pharmacol. 1995 May;115(1):39–46. doi: 10.1111/j.1476-5381.1995.tb16317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath M., Warren D. J., Sanderson C. J. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985 Nov 7;83(2):209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- Tomkinson A., Karlsson J. A., Raeburn D. Comparison of the effects of selective inhibitors of phosphodiesterase types III and IV in airway smooth muscle with differing beta-adrenoceptor subtypes. Br J Pharmacol. 1993 Jan;108(1):57–61. doi: 10.1111/j.1476-5381.1993.tb13439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem B. J., Meeker S. N., Chen J. Inhibition of neurally mediated nonadrenergic, noncholinergic contractions of guinea pig bronchus by isozyme-selective phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1994 Nov;271(2):811–817. [PubMed] [Google Scholar]
- Underwood D. C., Osborn R. R., Novak L. B., Matthews J. K., Newsholme S. J., Undem B. J., Hand J. M., Torphy T. J. Inhibition of antigen-induced bronchoconstriction and eosinophil infiltration in the guinea pig by the cyclic AMP-specific phosphodiesterase inhibitor, rolipram. J Pharmacol Exp Ther. 1993 Jul;266(1):306–313. [PubMed] [Google Scholar]


