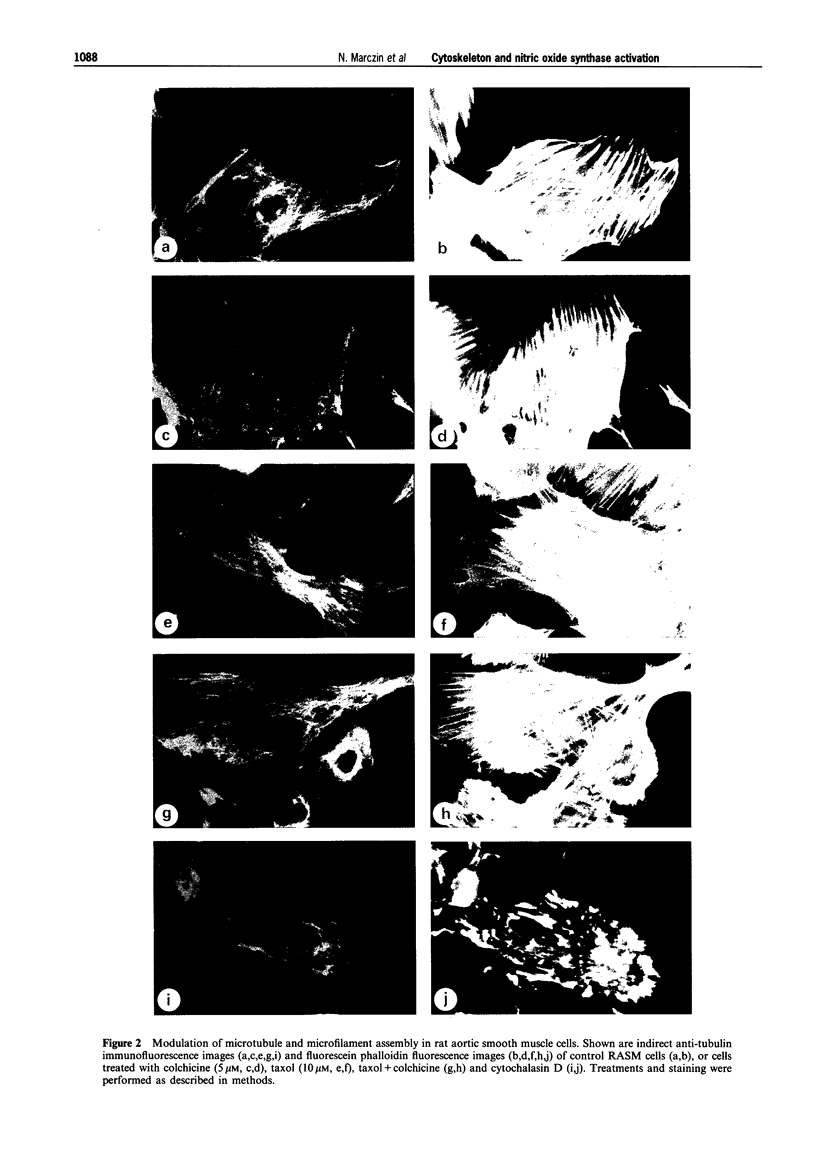
Abstract
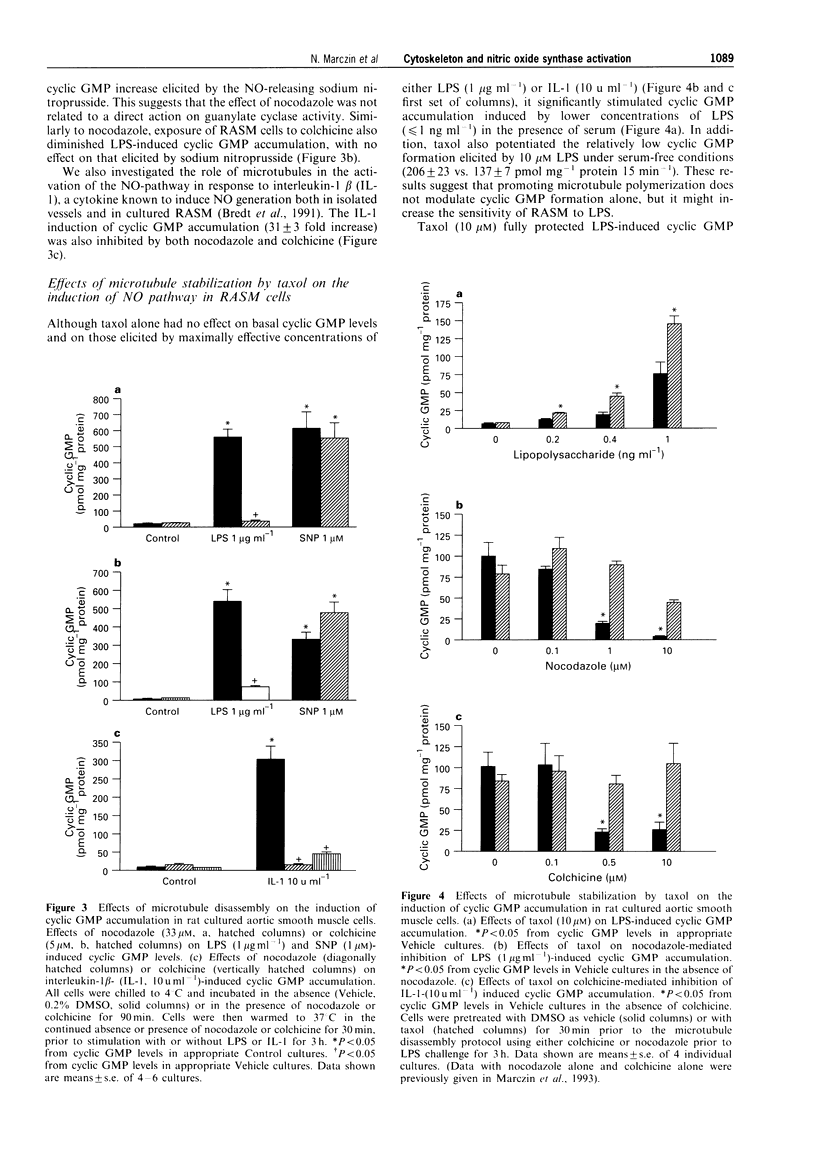
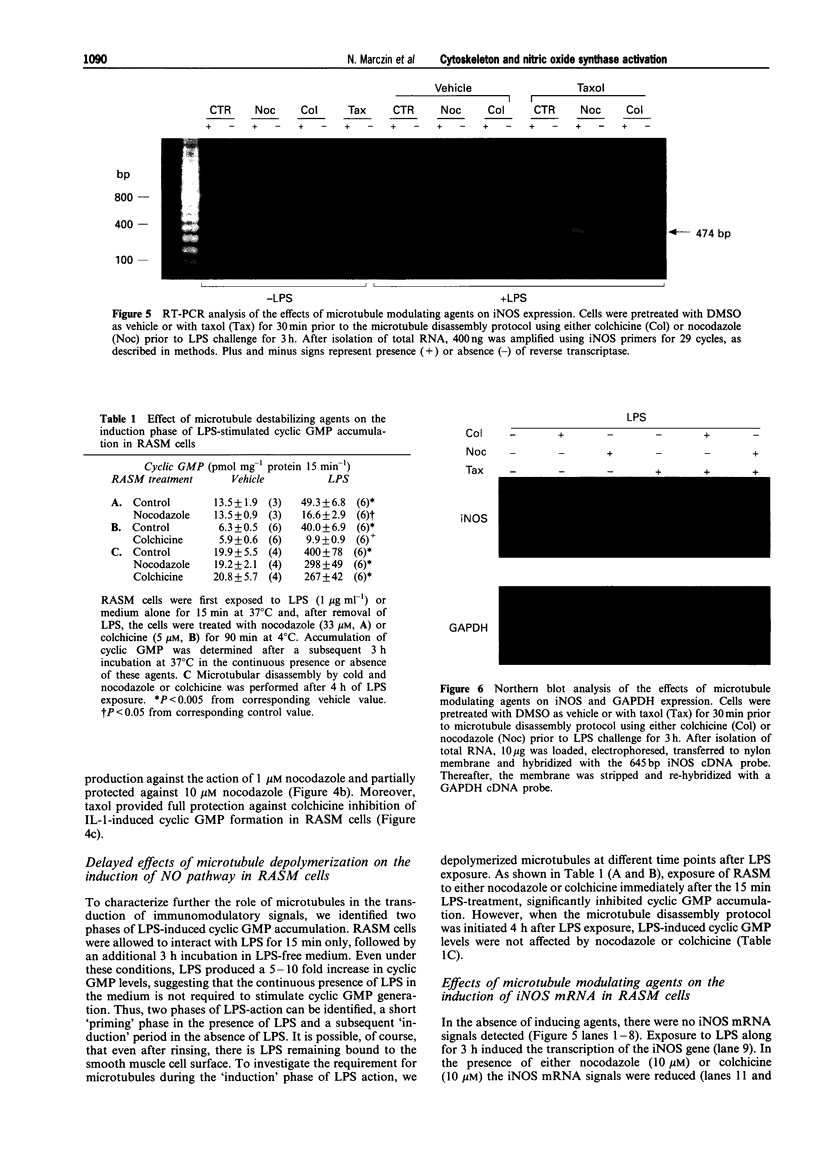
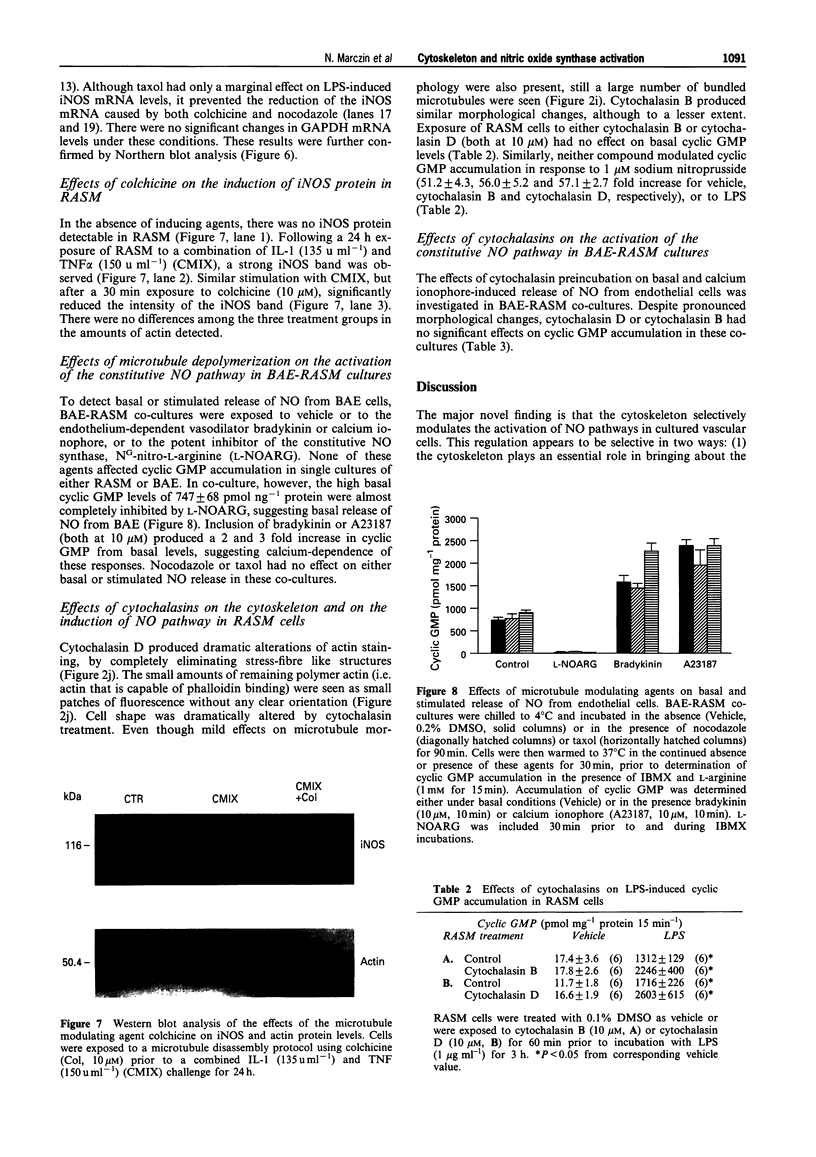
1. Vascular endothelial and smooth muscle cells generate nitric oxide (NO) via different nitric oxide synthase (NOS) isozymes. Activation of the endothelial constitutive NOS (ecNOS) contributes to the maintenance of cardiovascular homeostasis, whereas expression of the endotoxin- and cytokine-inducible pathway (iNOS) within the vascular smooth muscle is thought to be responsible for the cardiovascular collapse which occurs during septic shock and antitumour therapy with cytokines. Since the cytoskeleton is involved in the activation of certain genes and in some effects of endotoxin in macrophages, we investigated the role of microtubules and microfilaments in the activation of the NO pathway in cultured vascular cells. 2. Depolymerization of microtubules by either nocodazole or colchicine prevented lipopolysaccharide (LPS)- and interleukin-1 beta-induction of NO-dependent cyclic GMP accumulation. Steady state levels of iNOS mRNA, assessed by Northern blot and RT-PCR, and iNOS protein, assessed by Western blotting, were also decreased by either colchicine or nocodazole treatment. 3. Taxol enhanced microtubule polymerization alone, and prevented microtubule depolymerization elicited by nocodazole and colchicine. Associated with its effect on microtubule assembly, taxol prevented the inhibitory effects of nocodazole and colchicine on cyclic GMP accumulation and iNOS mRNA levels. 4. Disruption of microfilaments by cytochalasins had no inhibitory effect on the activation of the inducible NO pathway. 5. In contrast to cytokine-stimulated smooth muscle cells, modulation of either microtubule or microfilament assembly did not affect the constitutive NO pathway in endothelial cells, as endothelial cell- and NO-dependent cyclic GMP accumulation in endothelial-smooth muscle co-cultures remained unchanged. 6. Our findings demonstrate that microtubules play a prominent role in the activation of the inducible NO pathway in response to inflammatory mediators in smooth muscle cells but not of the constitutive synthesis of NO in endothelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley D., Cohen R. A., Levinsky N. G. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989 Jan;83(1):331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D. Interleukin 1 and endotoxin activate soluble guanylate cyclase in vascular smooth muscle. Am J Physiol. 1990 Jul;259(1 Pt 2):R38–R44. doi: 10.1152/ajpregu.1990.259.1.R38. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri F. M., Ballmer-Hofer K., Rajput B., Nagamine Y. Disruption of cytoskeletal structures results in the induction of the urokinase-type plasminogen activator gene expression. J Biol Chem. 1990 Aug 5;265(22):13327–13334. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., McKinnon W. C., Mostov K. E. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 1):2365–2373. doi: 10.1083/jcb.111.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Cooper R., Papaconstantinou J., Ritchie D. G. Microtubule depolymerization inhibits the regulation of alpha 1-acid glycoprotein mRNA by hepatocyte stimulating factor. J Biol Chem. 1989 Jan 5;264(1):515–519. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M., Goldfinger S. E., Dale D. C., Alling D. W. Colchicine therapy for familial mediterranean fever. A double-blind trial. N Engl J Med. 1974 Oct 31;291(18):934–937. doi: 10.1056/NEJM197410312911804. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents. J Exp Med. 1990 Mar 1;171(3):715–727. doi: 10.1084/jem.171.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990 Apr 20;248(4953):370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland D. L. Kinetics and mechanism of colchicine binding to tubulin: evidence for ligand-induced conformational change. Biochemistry. 1978 Oct 3;17(20):4266–4272. doi: 10.1021/bi00613a024. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Geroulanos S., Schilling J., Cakmakci M., Jung H. H., Largiader F. Inhibition of NO synthesis in septic shock. Lancet. 1992 Feb 15;339(8790):435–435. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., O'Shaughnessy K., Moore K., Ramrakha P., Wilkins M. R. Induction of nitric oxide synthase in cultured vascular smooth muscle cells: the role of cyclic AMP. Br J Pharmacol. 1994 Jun;112(2):396–402. doi: 10.1111/j.1476-5381.1994.tb13085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Ishii K., Sheng H., Warner T. D., Förstermann U., Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am J Physiol. 1991 Aug;261(2 Pt 2):H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Field D. J., Lee L. L. Effects of nocodazole on structures of calf brain tubulin. Biochemistry. 1980 Dec 23;19(26):6209–6215. doi: 10.1021/bi00567a041. [DOI] [PubMed] [Google Scholar]
- Loeb A. L., Owens G. K., Peach M. J. Evidence for endothelium-derived relaxing factor in cultured cells. Hypertension. 1985 Sep-Oct;7(5):804–807. doi: 10.1161/01.hyp.7.5.804. [DOI] [PubMed] [Google Scholar]
- Malawista S. E. Colchicine: a common mechanism for its anti-inflammatory and anti-mitotic effects. Arthritis Rheum. 1968 Apr;11(2):191–197. doi: 10.1002/art.1780110210. [DOI] [PubMed] [Google Scholar]
- Malkinson F. D. Colchicine. New uses of an old, old drug. Arch Dermatol. 1982 Jul;118(7):453–457. doi: 10.1001/archderm.118.7.453. [DOI] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Jilling T., Catravas J. D. Prevention of nitric oxide synthase induction in vascular smooth muscle cells by microtubule depolymerizing agents. Br J Pharmacol. 1993 Jul;109(3):603–605. doi: 10.1111/j.1476-5381.1993.tb13613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczin N., Ryan U. S., Catravas J. D. Effects of oxidant stress on endothelium-derived relaxing factor-induced and nitrovasodilator-induced cGMP accumulation in vascular cells in culture. Circ Res. 1992 Feb;70(2):326–340. doi: 10.1161/01.res.70.2.326. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moritz T., Niederle N., Baumann J., May D., Kurschel E., Osieka R., Kempeni J., Schlick E., Schmidt C. G. Phase I study of recombinant human tumor necrosis factor alpha in advanced malignant disease. Cancer Immunol Immunother. 1989;29(2):144–150. doi: 10.1007/BF00199290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Otto A. M., Zumbé A., Gibson L., Kubler A. M., Jimenez de Asua L. Cytoskeleton-disrupting drugs enhance effect of growth factors and hormones on initiation of DNA synthesis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6435–6438. doi: 10.1073/pnas.76.12.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984 Jun;56:103–114. doi: 10.1289/ehp.8456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santell L., Marotti K., Bartfeld N. S., Baynham P., Levin E. G. Disruption of microtubules inhibits the stimulation of tissue plasminogen activator expression and promotes plasminogen activator inhibitor type 1 expression in human endothelial cells. Exp Cell Res. 1992 Aug;201(2):358–365. doi: 10.1016/0014-4827(92)90284-f. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Catovsky S., Durante W., Scott-Burden T., Schafer A. I., Vanhoutte P. M. Thrombin inhibits induction of nitric oxide synthase in vascular smooth muscle cells. Am J Physiol. 1993 Feb;264(2 Pt 2):H611–H616. doi: 10.1152/ajpheart.1993.264.2.H611. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991 Sep 13;253(5025):1275–1277. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986 Sep;103(3):1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Whitfield J. F. Cytoplasmic microtubules are essential for the formation of membrane-bound polyribosomes. J Biol Chem. 1985 Jan 25;260(2):765–770. [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]