Abstract
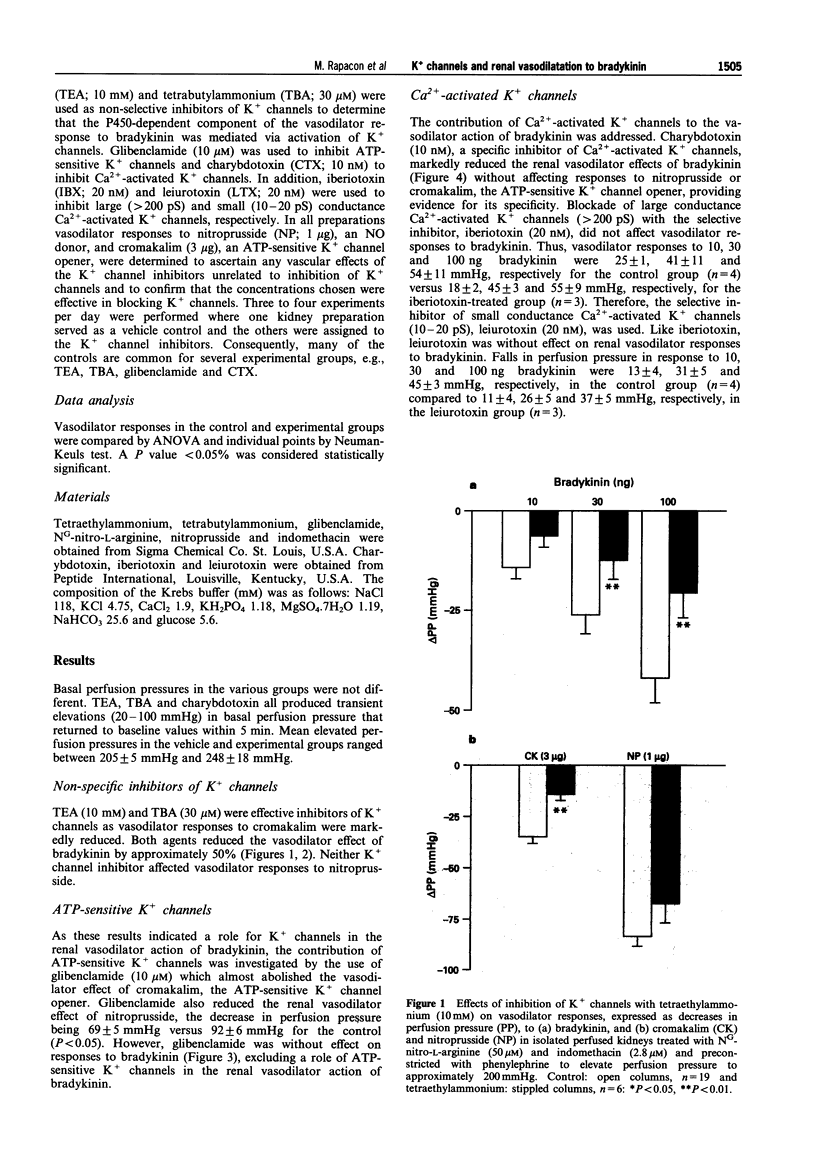
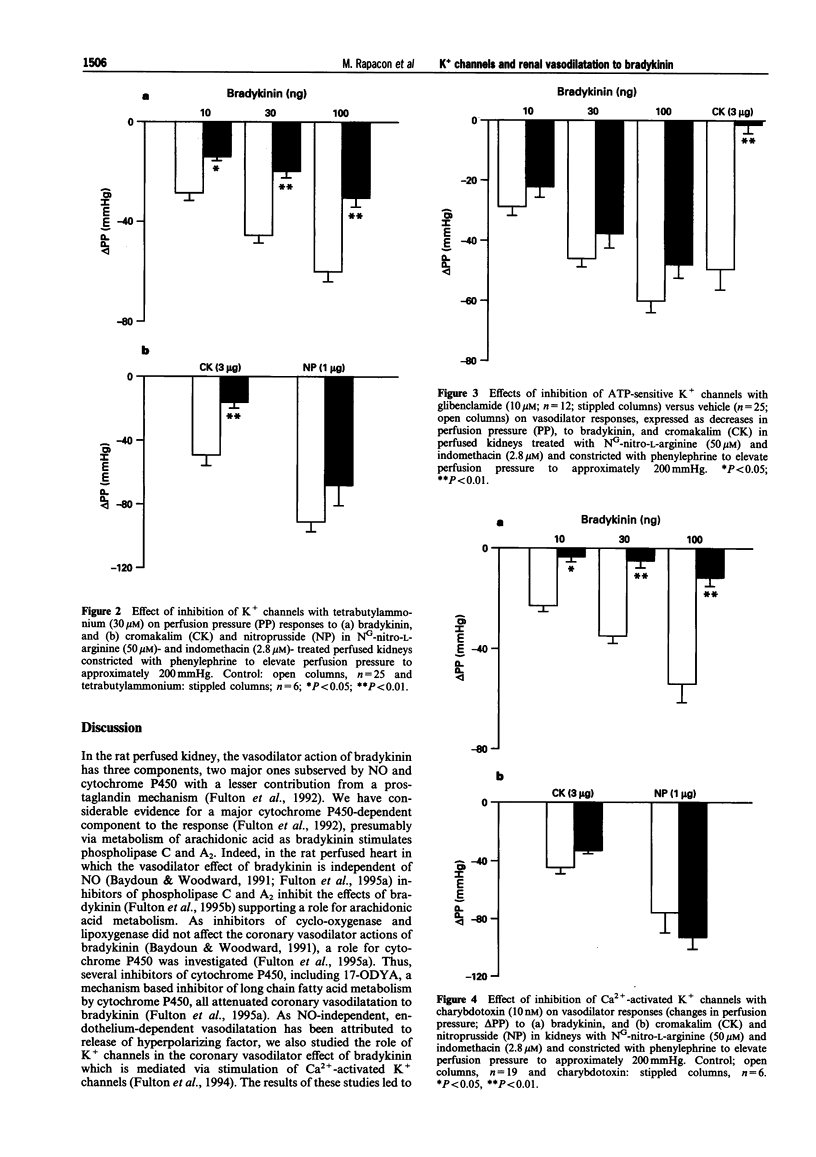
1. NO- and prostaglandin-independent, endothelium-dependent vasodilator responses to bradykinin are attributed to release of a hyperpolarizing factor. Therefore, the contribution of K+ channels to the renal vasodilator effect of bradykinin was examined in rat perfused kidneys that were preconstricted with phenylephrine and treated with NG-nitro-L-arginine (L-NOARG) and indomethacin to inhibit NO and prostaglandin synthesis. 2. The non-specific K+ channel inhibitors, TEA and TBA reduced vasodilator responses to bradykinin and cromakalim but not those to nitroprusside. 3. Glibenclamide, an inhibitor of ATP-sensitive K+ channels, blocked the vasodilator response to cromakalim without affecting responses to bradykinin. 4. Charybdotoxin, a selective inhibitor of Ca(2+)-activated K+ channels, greatly attenuated vasodilator responses to bradykinin without affecting those to cromakalim or nitroprusside. 5. Iberiotoxin and leiurotoxin, inhibitors of large and small conductance Ca(2+)-activated K+ channels, respectively, were without effect on vasodilator responses to bradykinin, cromakalim or nitroprusside. 6. These results implicate K+ channels, specifically Ca(2+)-activated K+ channels of intermediate conductance, in the renal vasodilator effect of bradykinin and, thereby, support a role for a hyperpolarizing factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baydoun A. R., Woodward B. Effects of bradykinin in the rat isolated perfused heart: role of kinin receptors and endothelium-derived relaxing factor. Br J Pharmacol. 1991 Jul;103(3):1829–1833. doi: 10.1111/j.1476-5381.1991.tb09871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [PubMed] [Google Scholar]
- Cachofeiro V., Nasjletti A. Increased vascular responsiveness to bradykinin in kidneys of spontaneously hypertensive rats. Effect of N omega-nitro-L-arginine. Hypertension. 1991 Nov;18(5):683–688. doi: 10.1161/01.hyp.18.5.683. [DOI] [PubMed] [Google Scholar]
- Chicchi G. G., Gimenez-Gallego G., Ber E., Garcia M. L., Winquist R., Cascieri M. A. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. J Biol Chem. 1988 Jul 25;263(21):10192–10197. [PubMed] [Google Scholar]
- Cowan C. L., Cohen R. A. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am J Physiol. 1991 Sep;261(3 Pt 2):H830–H835. doi: 10.1152/ajpheart.1991.261.3.H830. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Mahboubi K., McGiff J. C., Quilley J. Cytochrome P450-dependent effects of bradykinin in the rat heart. Br J Pharmacol. 1995 Jan;114(1):99–102. doi: 10.1111/j.1476-5381.1995.tb14911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., McGiff J. C., Quilley J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br J Pharmacol. 1992 Nov;107(3):722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., McGiff J. C., Quilley J. Role of K+ channels in the vasodilator response to bradykinin in the rat heart. Br J Pharmacol. 1994 Nov;113(3):954–958. doi: 10.1111/j.1476-5381.1994.tb17085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Kitamura K., Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995 Sep;47(3):387–573. [PubMed] [Google Scholar]
- Oyekan A. O., McGiff J. C., Rosencrantz-Weiss P., Quilley J. Relaxant responses of rabbit aorta: influence of cytochrome P450 inhibitors. J Pharmacol Exp Ther. 1994 Jan;268(1):262–269. [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]



