Abstract
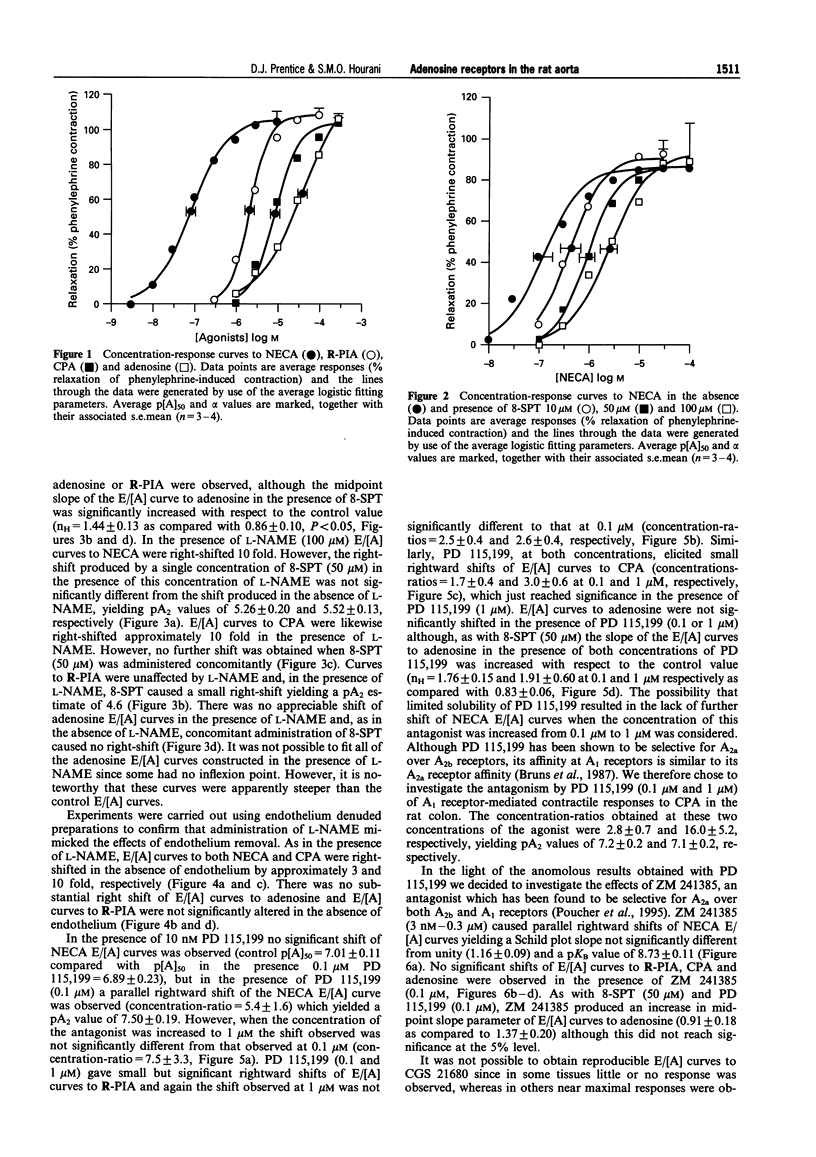
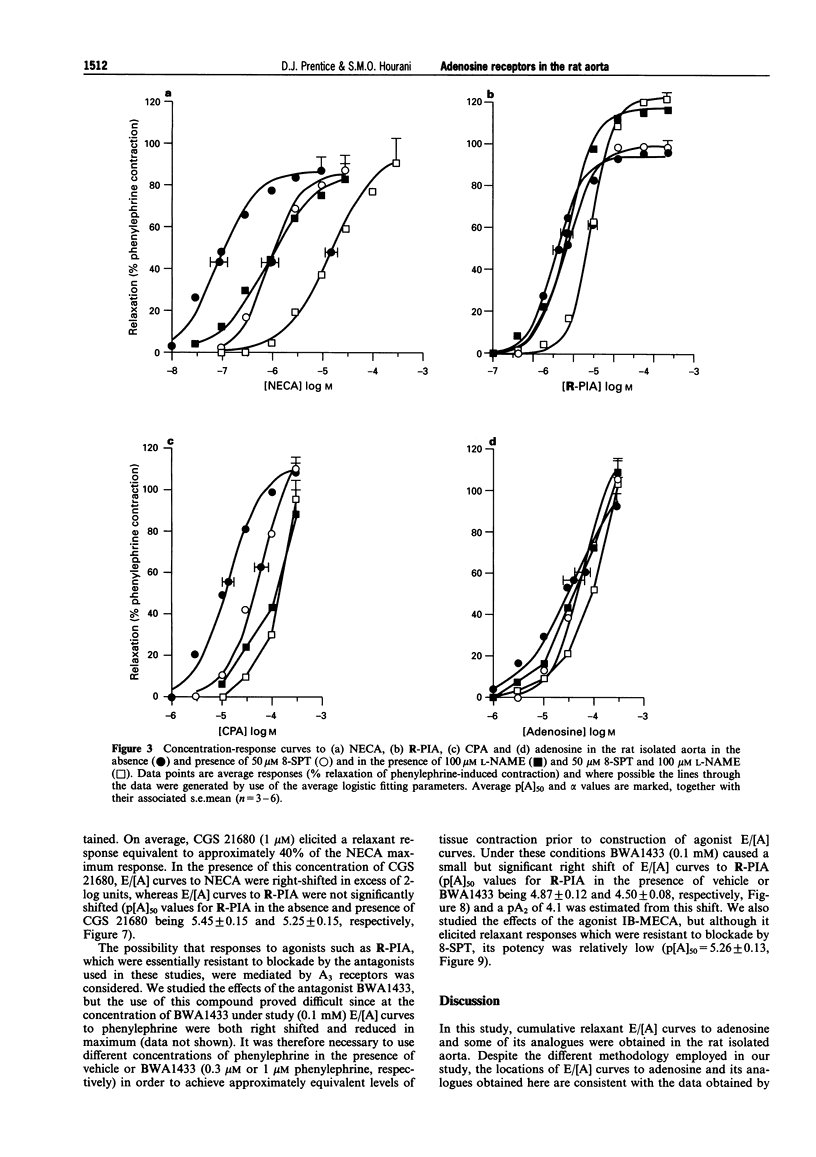
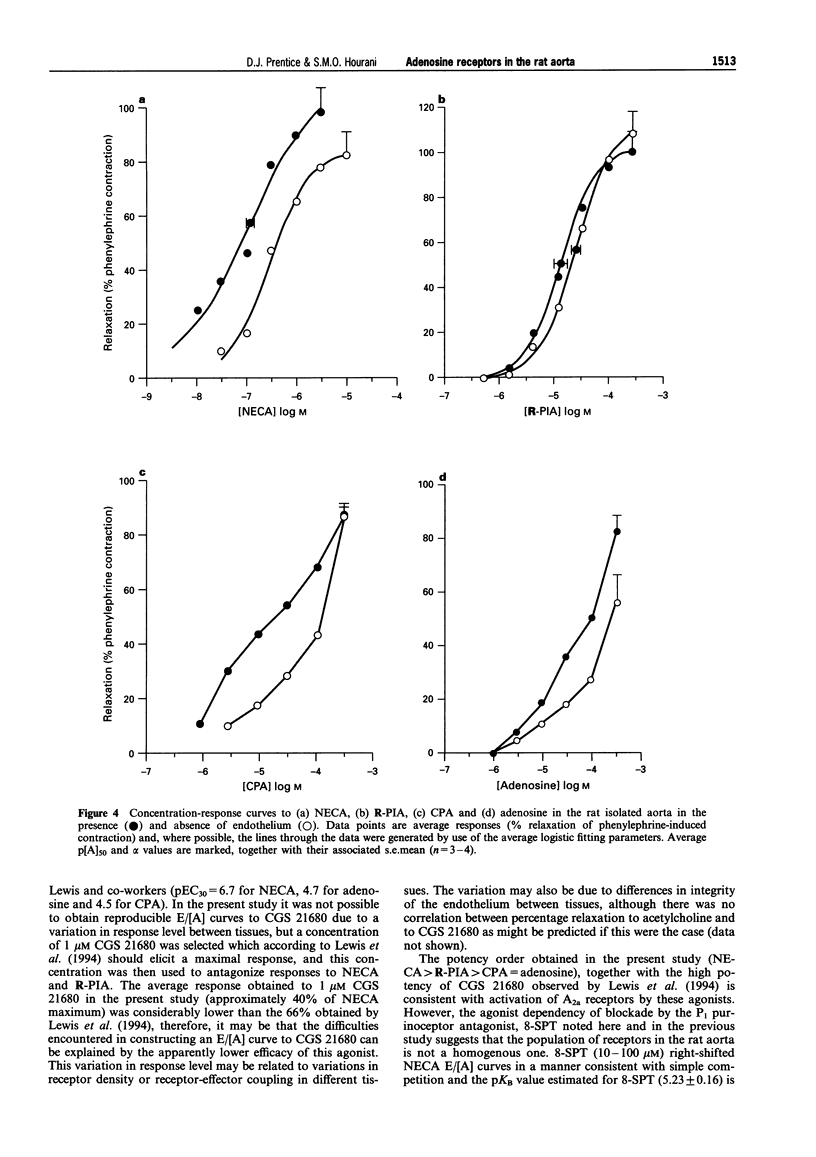
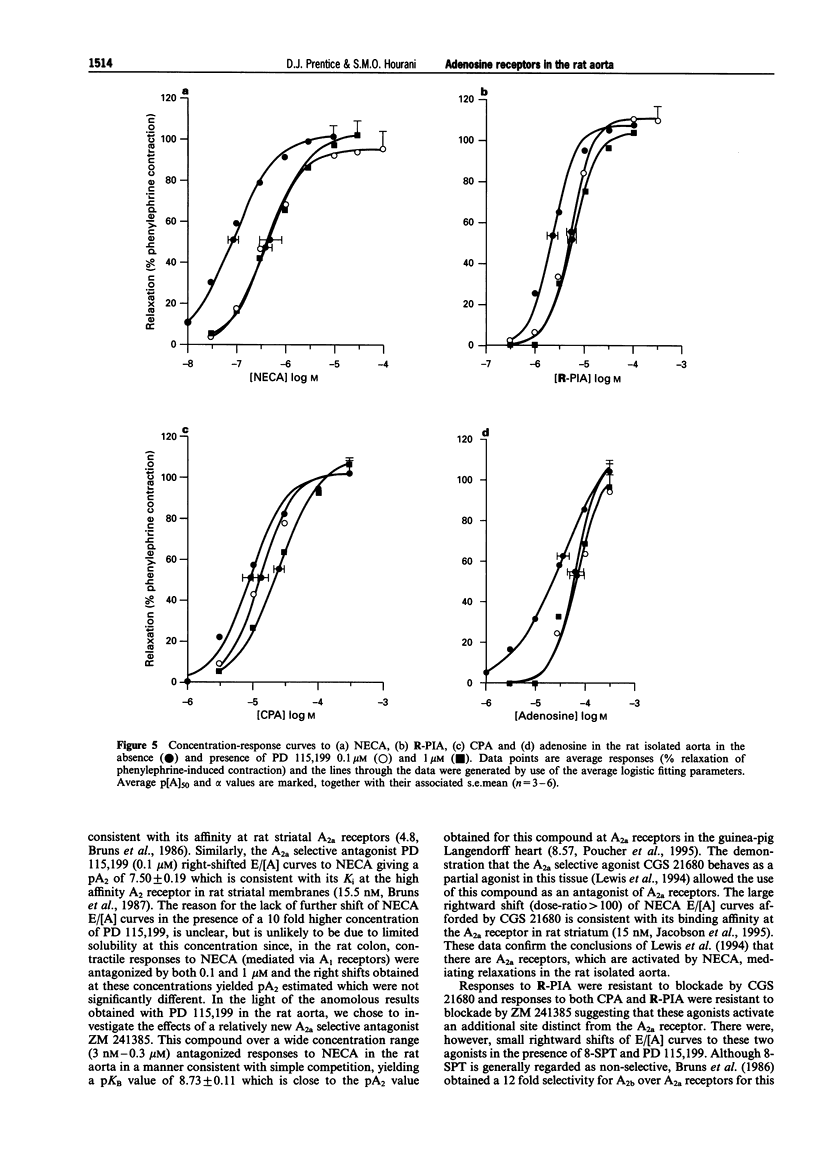
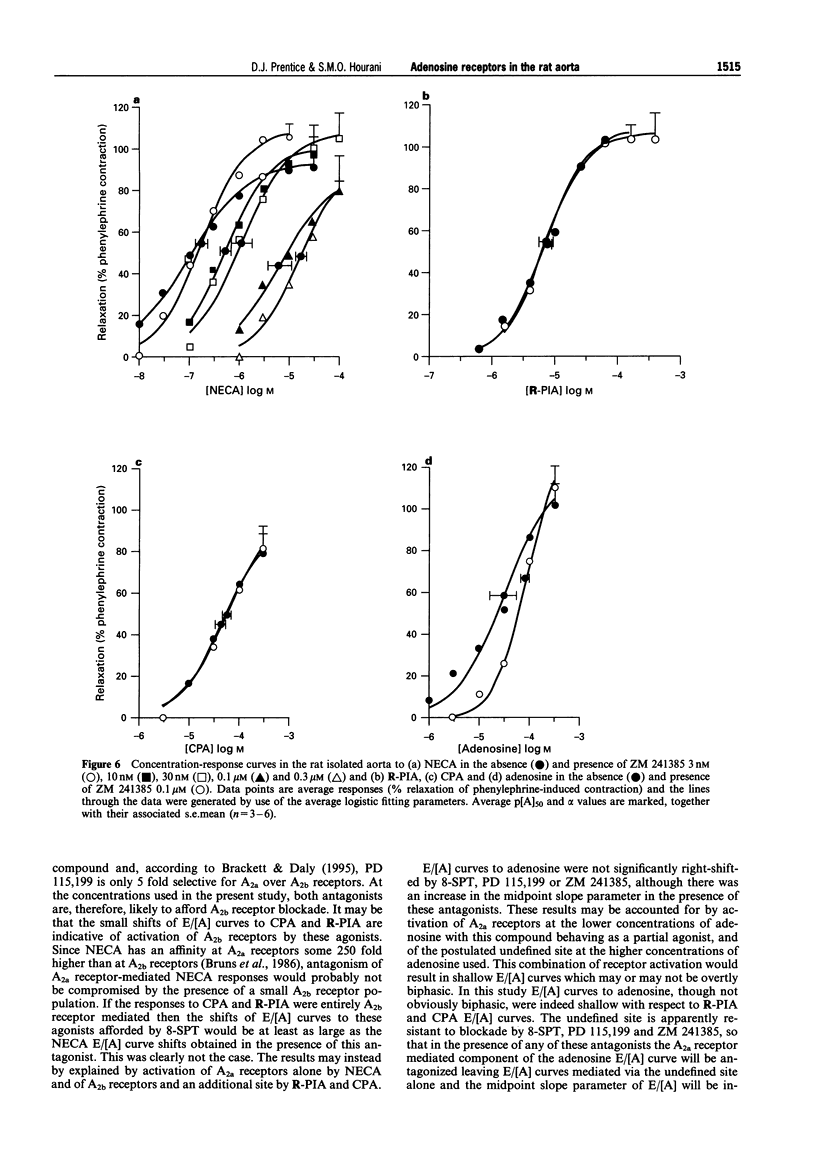
1. The presence of A2 receptors mediating relaxation in the rat isolated aorta has been previously demonstrated. However, agonist dependency of the degree of rightward shift elicited by 8-sulphophenyltheophylline (8-SPT) led to the suggestion that the population of receptors in this tissue is not a homogeneous one. In this study we have re-examined the effects of 8-SPT in the absence and presence of the NO synthase inhibitor L-NAME (NG-nitro-L-arginine methyl ester) and investigated antagonism of responses by the potent A2a receptor ligands PD 115,199 (N-[2-dimethylamino)ethyl]-N-methyl-4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3 dipropyl-1H-purin-8-yl)) benzene sulphonamidexanthine), ZM 241385 (4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-yl amino]ethyl)phenol), and CGS 21680 (2-[p-(2-carboxyethyl)phenylamino]-5'-N-ethylcarboxamidoadenosine). We have also investigated the antagonist effects of BWA1433 (1,3-dipropyl-8-(4-acrylate)phenylxanthine) which has been shown to have affinity at rat A3 receptors. 2. Adenosine, R-PIA (N6-R-phenylisopropyl adenosine), CPA (N6-cyclopentyladenosine) and NECA (5'-N-ethylcarboxamidoadenosine) all elicited relaxant responses in the phenylephrine pre-contracted rat isolated aorta with the following potency order (p[A50] values in parentheses): NECA (7.07 +/- 0.11) > R-PIA (5.65 +/- 0.10) > CPA (5.05 +/- 0.12) > adenosine (4.44 +/- 0.12). 3. 8-SPT (10-100 microM) caused parallel rightward shifts of the E/[A] curves to NECA (pKB = 5.23 +/- 0.16). A smaller rightward shift of E/[A] curves to CPA was observed (pA2 = 4.85 +/- 0.17). However, no significant shifts of E/[A] curves to either adenosine or R-PIA were observed. 4. In the absence of endothelium E/[A] curves to NECA and CPA were right-shifted compared to controls. However, removal of the endothelium did not produce a substantial shift of adenosine E/[A] curves, and E/[A] curves to R-PIA were unaffected by removal of the endothelium. 5. In the presence of L-NAME (100 microM) E/[A] curves to NECA and CPA were right-shifted. However, no further shift of the CPA E/[A] curve was obtained when 8-SPT (50 microM) was administered concomitantly. The locations of curves to R-PIA and adenosine were unaffected by L-NAME (100 microM). 6. In the presence of PD 115,199 (0.1 microM) a parallel rightward shift of NECA E/[A] curves was observed (pA2 = 7.50 +/- 0.19). PD 115,199 (0.1 and 1 microM) gave smaller rightward shifts of E/[A] curves to R-PIA and CPA, but E/[A] curves to adenosine were not significantly shifted in the presence of PD 115,199 (0.1 or 1 microM). 7. The presence of ZM 241385 (3 nM-0.3 microM) caused parallel rightwad shifts of NECA E/[A] curves (pKB = 8.73 +/- 0.11). No significant shifts of E/[A] curves to adenosine, CPA or R-PIA were observed in the presence of 0.1 microM ZM 241385. 8. CGS 21680 (1 microM) elicited a relaxant response equivalent to approximately 40% of the NECA maximum response. In the presence of this concentration of CGS 21680, E/[A] curves to NECA were right-shifted in excess of 2-log units, whereas E/[A] curves to R-PIA were not significantly shifted. 9. BWA1433 (100 microM) caused a small but significant right-shift of the E/[A] curve to R-PIA yielding a pA2 estimate of 4.1 IB-MECA (N6-(3-iodo-benzyl)adenosine-5(1)-N-methyl uronamide) elicited relaxant responses which were resistant to blockade by 8-SPT (p[A]50 = 5.26 +/- 0.13). 10. The results suggest that whereas relaxations to NECA (10 nM-1 microM) are mediated via adenosine A2a receptors, which are located at least in part on the endothelium, R-PIA and CPA may activate A2b receptors on the endothelium and an additional, as yet undefined site, which is likely to be located on the smooth muscle and which is not susceptible to blockade by 8-SPT, PD 115,199 or ZM 241385. This site is unlikely to be an A3 receptor since the very small shift obtained in the presence of BWA1433 (100 microM), and the low potency of IB-MECA is not consistent with the affin
Full text
PDF

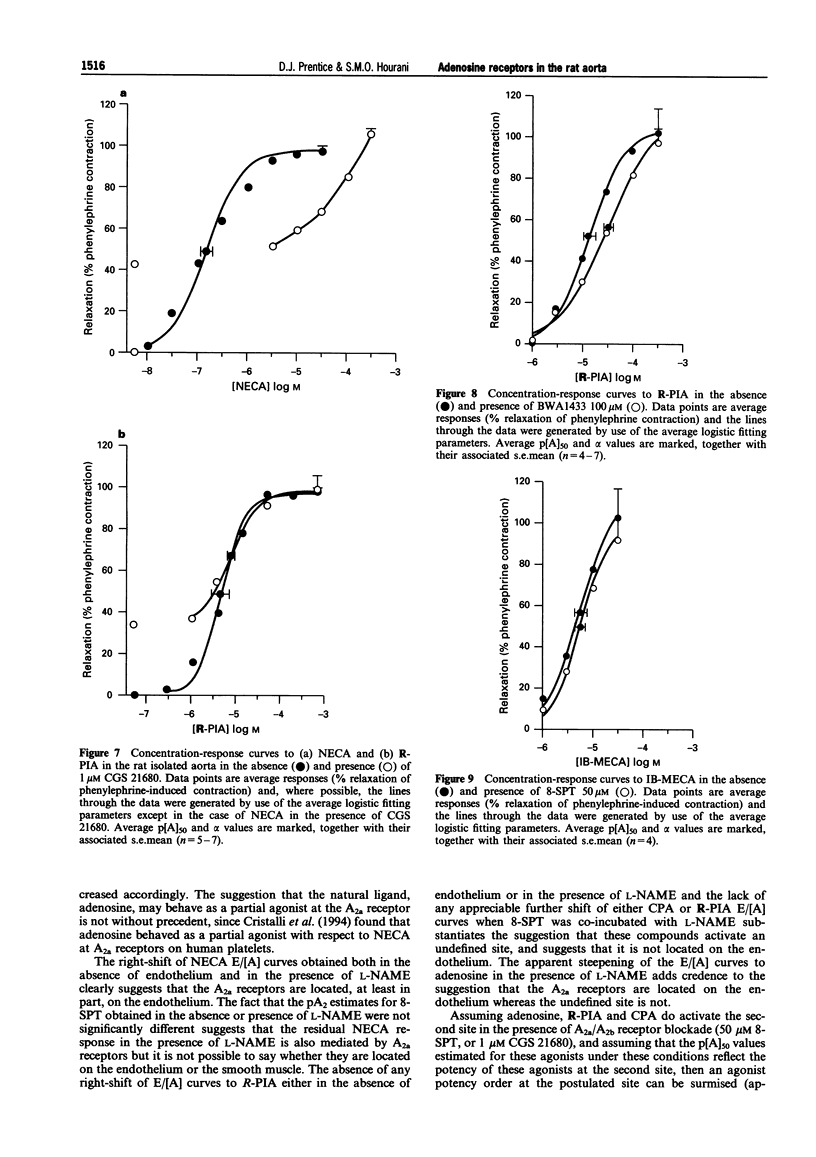

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey S. J., Hourani S. M. Effects of purines on the longitudinal muscle of the rat colon. Br J Pharmacol. 1992 Apr;105(4):885–892. doi: 10.1111/j.1476-5381.1992.tb09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Shankley N. P. The isolated stomach preparation of the mouse: a physiological unit for pharmacological analysis. Br J Pharmacol. 1985 Nov;86(3):571–579. doi: 10.1111/j.1476-5381.1985.tb08933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett L. E., Daly J. W. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994 Mar 2;47(5):801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- Brackett L. E., Daly J. W. Relaxant effects of adenosine analogs on guinea pig trachea in vitro: xanthine-sensitive and xanthine-insensitive mechanisms. J Pharmacol Exp Ther. 1991 Apr;257(1):205–213. [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hays S. J. PD 115,199: an antagonist ligand for adenosine A2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Brown C. M. Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol. 1983 Dec 9;96(1-2):61–69. doi: 10.1016/0014-2999(83)90529-0. [DOI] [PubMed] [Google Scholar]
- Cristalli G., Vittori S., Thompson R. D., Padgett W. L., Shi D., Daly J. W., Olsson R. A. Inhibition of platelet aggregation by adenosine receptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1994 Jun;349(6):644–650. doi: 10.1007/pl00004904. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Hourani S. M., Long C. J., Collis M. G. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol. 1994 Nov;25(7):1381–1387. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. L. Relative agonist potencies of C2-substituted analogues of adenosine: evidence for adenosine A2B receptors in the guinea pig aorta. Eur J Pharmacol. 1992 Jun 5;216(2):235–242. doi: 10.1016/0014-2999(92)90365-b. [DOI] [PubMed] [Google Scholar]
- Moritoki H., Matsugi T., Takase H., Ueda H., Tanioka A. Evidence for the involvement of cyclic GMP in adenosine-induced, age-dependent vasodilatation. Br J Pharmacol. 1990 Jul;100(3):569–575. doi: 10.1111/j.1476-5381.1990.tb15848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher S. M., Keddie J. R., Singh P., Stoggall S. M., Caulkett P. W., Jones G., Coll M. G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995 Jul;115(6):1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice D. J., Shankley N. P., Black J. W. Pharmacological analysis of the interaction between purinoceptor agonists and antagonists in the guinea-pig taenia caecum. Br J Pharmacol. 1995 Jun;115(4):549–556. doi: 10.1111/j.1476-5381.1995.tb14967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose'Meyer R. B., Hope W. Evidence that A2 purinoceptors are involved in endothelium-dependent relaxation of the rat thoracic aorta. Br J Pharmacol. 1990 Jul;100(3):576–580. doi: 10.1111/j.1476-5381.1990.tb15849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. L., Linden J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc Res. 1993 Jan;27(1):62–67. doi: 10.1093/cvr/27.1.62. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Li C., Olah M. E., Johnson R. A., Stiles G. L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


