Abstract
1. The release of somatostatin-like immunoreactivity (SRIF-LI) in the human brain was studied in synaptosomal preparations from fresh neocortical specimens obtained from patients undergoing neurosurgery to remove deeply sited tumours. 2. The basal outflow of SRIF-LI from superfused synaptosomes was increased about 3 fold during exposure to a depolarizing medium containing 15 mM KCl. The K(+)-evoked overflow of SRIF-LI was almost totally dependent on the presence of Ca2+ in the superfusion medium. 3. The GABAB receptor agonist, (-)-baclofen (0.3 - 100 microM), inhibited the overflow of SRIF-LI in a concentration-dependent manner (EC50 = 1.84 +/- 0.20 microM; maximal effect: about 50%). The novel GABAB receptor ligand, 3-aminopropyl(difluoromethyl)phosphinic acid (CGP 47656) mimicked (-)-baclofen in inhibiting the SRIF-LI overflow (EC50 = 3.06 +/- 0.52 microM; maximal effect: about 50%), whereas the GABAA receptor agonist, muscimol, was ineffective up to 100 microM. 4. The inhibition by 10 microM (-)-baclofen of the K(+)-evoked SRIF-LI overflow was concentration-dependently prevented by two selective GABAB receptor antagonists, 3-amino-propyl (diethoxymethyl)-phosphinic acid (CGP 35348) (IC50 = 24.40 +/- 2.52 microM) and [3-[[(3,4-dichlorophenyl) methyl]amino]propyl] (diethoxymethyl) phosphinic acid (CGP 52432) (IC50 = 0.06 +/- 0.005 microM). 5. The inhibition of SRIF-LI overflow caused by 10 microM CGP 47656 was abolished by 1 microM CGP 52432. 6. When human synaptosomes were labelled with [3H]-GABA and depolarized in superfusion with 15 mM KCl, the inhibition by 10 microM (-)-baclofen of the depolarization-evoked [3H]-GABA overflow was largely prevented by 10 microM CGP 47656 which therefore behaved as an autoreceptor antagonist. 7. In conclusion: (a) the characteristics of SRIF-LI release from synaptosomal preparations of human neocortex are compatible with a neuronal origin; (b) the nerve terminals releasing the neuropeptide possess inhibitory receptors of the GABAB type; (c) these receptors differ pharmacologically from the GABAB autoreceptors present on human neocortex nerve terminals since the latter have been shown to be CGP 35348-insensitive but can be blocked by CGP 47656.
Full text
PDF

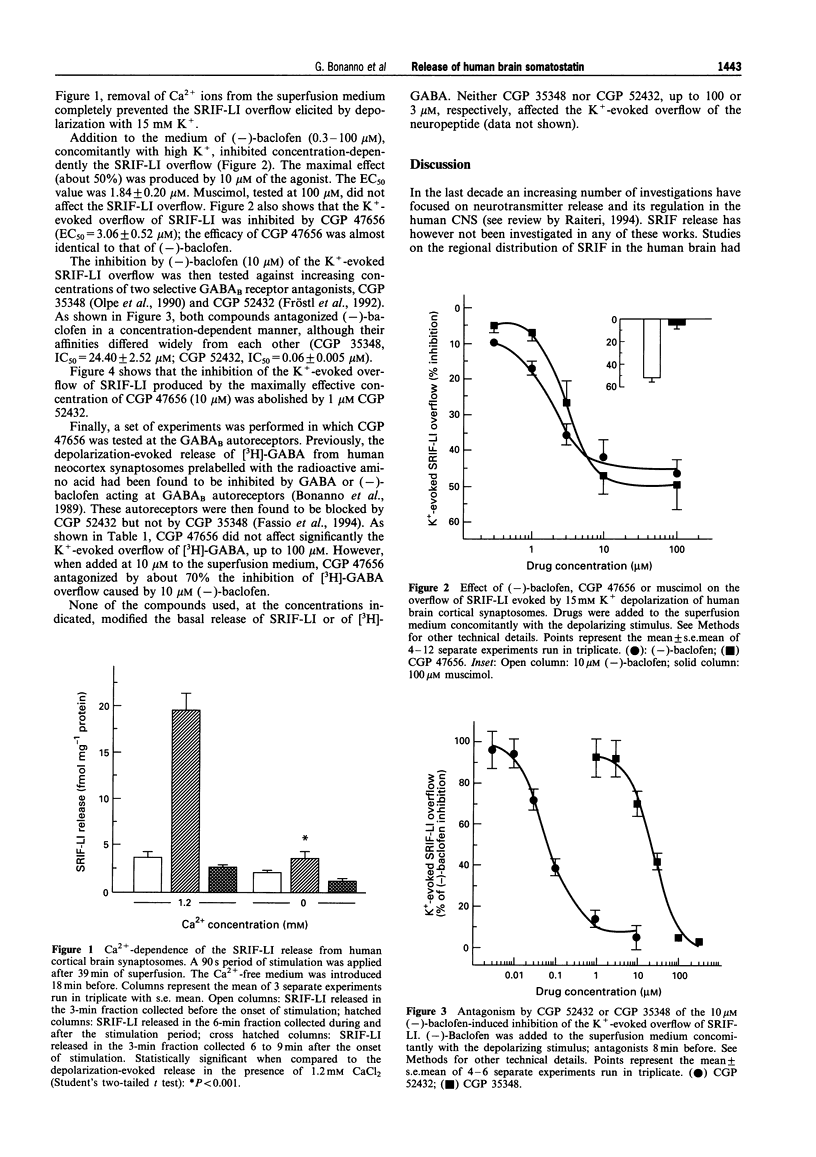
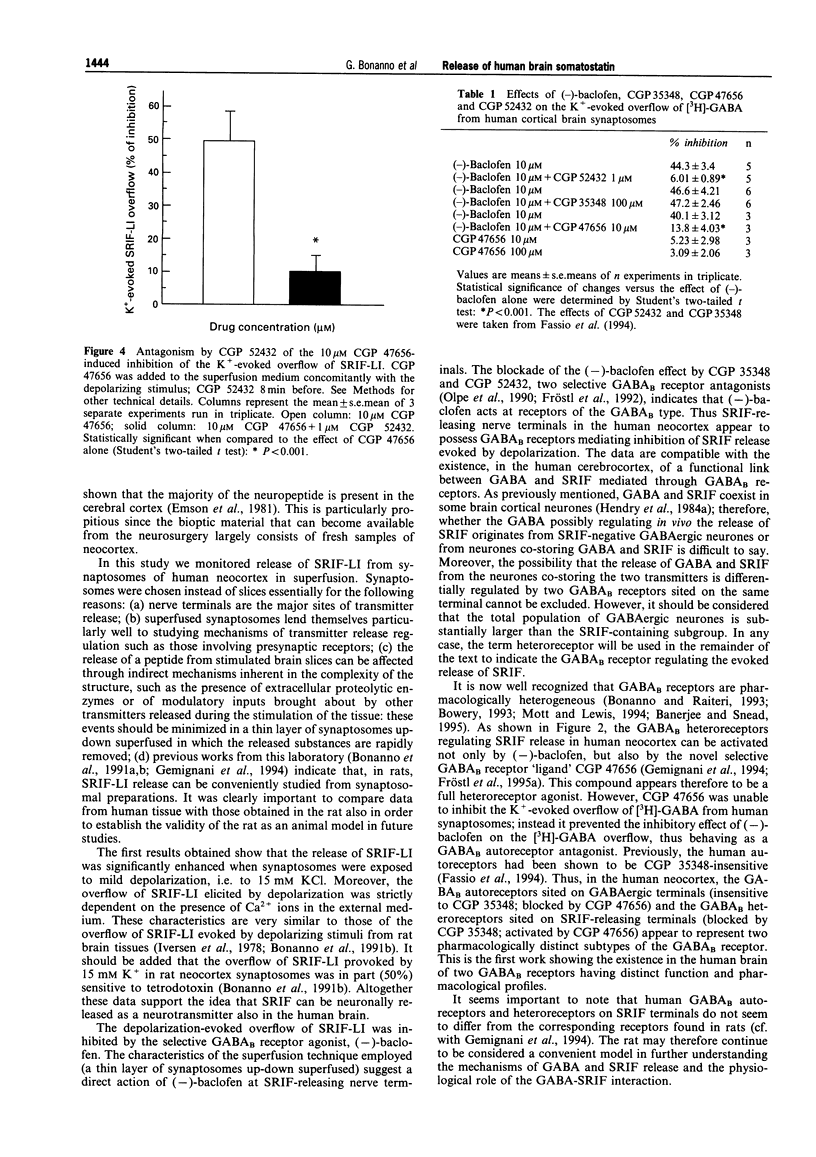


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee P. K., Snead O. C., 3rd Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB. J Pharmacol Exp Ther. 1995 Jun;273(3):1534–1543. [PubMed] [Google Scholar]
- Bennett G. W., Edwardson J. A., Marcano de Cotte M., Berelowitz M., Pimstone B. L., Kronheim S. Release of somatostatin from rat brain synaptosomes. J Neurochem. 1979 Mar;32(3):1127–1130. doi: 10.1111/j.1471-4159.1979.tb04606.x. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Cavazzani P., Andrioli G. C., Asaro D., Pellegrini G., Raiteri M. Release-regulating autoreceptors of the GABAB-type in human cerebral cortex. Br J Pharmacol. 1989 Feb;96(2):341–346. doi: 10.1111/j.1476-5381.1989.tb11823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G., Gemignani A., Fedele E., Fontana G., Raiteri M. gamma-Aminobutyric acidB receptors mediate inhibition of somatostatin release from cerebrocortex nerve terminals. J Pharmacol Exp Ther. 1991 Dec;259(3):1153–1157. [PubMed] [Google Scholar]
- Bonanno G., Parodi B., Cafaggi S., Raiteri M. Somatostatin release from rat cerebral cortex synaptosomes. J Neurochem. 1991 Oct;57(4):1258–1264. doi: 10.1111/j.1471-4159.1991.tb08287.x. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993 Jul;14(7):259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bowery N. G. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Somatostatin immunoreactive neurons in the human hippocampus and cortex shown by immunogold/silver intensification on vibratome sections: coexistence with neuropeptide Y neurons, and effects in Alzheimer-type dementia. J Comp Neurol. 1987 Jun 8;260(2):201–223. doi: 10.1002/cne.902600205. [DOI] [PubMed] [Google Scholar]
- Davies P., Katzman R., Terry R. D. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980 Nov 20;288(5788):279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Dawbarn D., De Quidt M. E., Emson P. C. Survival of basal ganglia neuropeptide Y-somatostatin neurones in Huntington's disease. Brain Res. 1985 Aug 12;340(2):251–260. doi: 10.1016/0006-8993(85)90921-7. [DOI] [PubMed] [Google Scholar]
- Dawbarn D., Rossor M. N., Mountjoy C. Q., Roth M., Emson P. C. Decreased somatostatin immunoreactivity but not neuropeptide Y immunoreactivity in cerebral cortex in senile dementia of Alzheimer type. Neurosci Lett. 1986 Sep 25;70(1):154–159. doi: 10.1016/0304-3940(86)90455-6. [DOI] [PubMed] [Google Scholar]
- DeNoble V. J., Hepler D. J., Barto R. A. Cysteamine-induced depletion of somatostatin produces differential cognitive deficits in rats. Brain Res. 1989 Mar 13;482(1):42–48. doi: 10.1016/0006-8993(89)90540-4. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Brazeau P., Tsang D., Brawer J., Martin J. B. Subcellular distribution of radioimmunoassayable somatostatin in rat brain. Brain Res. 1977 May 6;126(2):309–323. doi: 10.1016/0006-8993(77)90728-4. [DOI] [PubMed] [Google Scholar]
- Fassio A., Bonanno G., Cavazzani P., Raiteri M. Characterization of the GABA autoreceptor in human neocortex as a pharmacological subtype of the GABAB receptor. Eur J Pharmacol. 1994 Oct 3;263(3):311–314. doi: 10.1016/0014-2999(94)90727-7. [DOI] [PubMed] [Google Scholar]
- Froestl W., Mickel S. J., Hall R. G., von Sprecher G., Strub D., Baumann P. A., Brugger F., Gentsch C., Jaekel J., Olpe H. R. Phosphinic acid analogues of GABA. 1. New potent and selective GABAB agonists. J Med Chem. 1995 Aug 18;38(17):3297–3312. doi: 10.1021/jm00017a015. [DOI] [PubMed] [Google Scholar]
- Froestl W., Mickel S. J., von Sprecher G., Diel P. J., Hall R. G., Maier L., Strub D., Melillo V., Baumann P. A., Bernasconi R. Phosphinic acid analogues of GABA. 2. Selective, orally active GABAB antagonists. J Med Chem. 1995 Aug 18;38(17):3313–3331. doi: 10.1021/jm00017a016. [DOI] [PubMed] [Google Scholar]
- Gabriel S. M., Bierer L. M., Harotunian V., Purohit D. P., Perl D. P., Davis K. L. Widespread deficits in somatostatin but not neuropeptide Y concentrations in Alzheimer's disease cerebral cortex. Neurosci Lett. 1993 May 28;155(1):116–120. doi: 10.1016/0304-3940(93)90686-f. [DOI] [PubMed] [Google Scholar]
- Gemignani A., Paudice P., Bonanno G., Raiteri M. Pharmacological discrimination between gamma-aminobutyric acid type B receptors regulating cholecystokinin and somatostatin release from rat neocortex synaptosomes. Mol Pharmacol. 1994 Sep;46(3):558–562. [PubMed] [Google Scholar]
- Haroutunian V., Mantin R., Campbell G. A., Tsuboyama G. K., Davis K. L. Cysteamine-induced depletion of central somatostatin-like immunoactivity: effects on behavior, learning, memory and brain neurochemistry. Brain Res. 1987 Feb 17;403(2):234–242. doi: 10.1016/0006-8993(87)90060-6. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., DeFelipe J., Schmechel D., Brandon C., Emson P. C. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6526–6530. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., Emson P. C. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984 Oct;4(10):2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D., Lübbert H., Bruns C. Molecular pharmacology of somatostatin receptors. Naunyn Schmiedebergs Arch Pharmacol. 1994 Nov;350(5):441–453. doi: 10.1007/BF00173012. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Iversen S. D., Bloom F., Douglas C., Brown M., Vale W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nature. 1978 May 11;273(5658):161–163. doi: 10.1038/273161a0. [DOI] [PubMed] [Google Scholar]
- Manfridi A., Forloni G. L., Vezzani A., Fodritto F., De Simoni M. G. Functional and histological consequences of quinolinic and kainic acid-induced seizures on hippocampal somatostatin neurons. Neuroscience. 1991;41(1):127–135. doi: 10.1016/0306-4522(91)90203-z. [DOI] [PubMed] [Google Scholar]
- Marksteiner J., Sperk G. Concomitant increase of somatostatin, neuropeptide Y and glutamate decarboxylase in the frontal cortex of rats with decreased seizure threshold. Neuroscience. 1988 Aug;26(2):379–385. doi: 10.1016/0306-4522(88)90155-8. [DOI] [PubMed] [Google Scholar]
- Mondadori C., Jaekel J., Preiswerk G. CGP 36742: the first orally active GABAB blocker improves the cognitive performance of mice, rats, and rhesus monkeys. Behav Neural Biol. 1993 Jul;60(1):62–68. doi: 10.1016/0163-1047(93)90729-2. [DOI] [PubMed] [Google Scholar]
- Mott D. D., Lewis D. V. The pharmacology and function of central GABAB receptors. Int Rev Neurobiol. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Angelini F., Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol. 1974 Mar;25(3):411–414. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- Raiteri M. Functional studies of neurotransmitter receptors in human brain. Life Sci. 1994;54(22):1635–1647. doi: 10.1016/0024-3205(94)00604-0. [DOI] [PubMed] [Google Scholar]
- Reisine T., Bell G. I. Molecular properties of somatostatin receptors. Neuroscience. 1995 Aug;67(4):777–790. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- Schettini G. Brain somatostatin: receptor-coupled transducing mechanisms and role in cognitive functions. Pharmacol Res. 1991 Apr;23(3):203–215. doi: 10.1016/s1043-6618(05)80080-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., Smith A. D., Nunzi M. G., Gorio A., Wu J. Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984 Oct;4(10):2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Monno A., Rizzi M., Galli A., Barrios M., Samanin R. Somatostatin release is enhanced in the hippocampus of partially and fully kindled rats. Neuroscience. 1992 Nov;51(1):41–46. doi: 10.1016/0306-4522(92)90468-h. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Ruiz R., Monno A., Rizzi M., Lindefors N., Samanin R., Brodin E. Extracellular somatostatin measured by microdialysis in the hippocampus of freely moving rats: evidence for neuronal release. J Neurochem. 1993 Feb;60(2):671–677. doi: 10.1111/j.1471-4159.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]


