Abstract
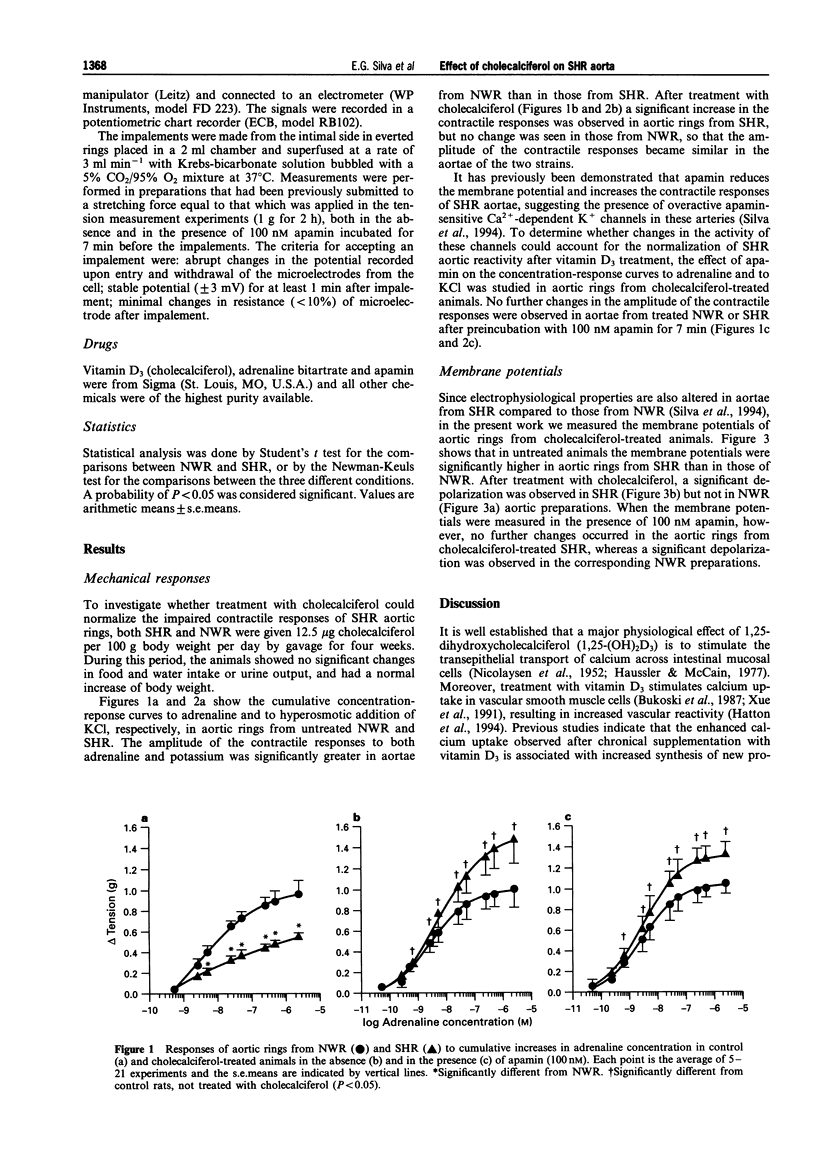
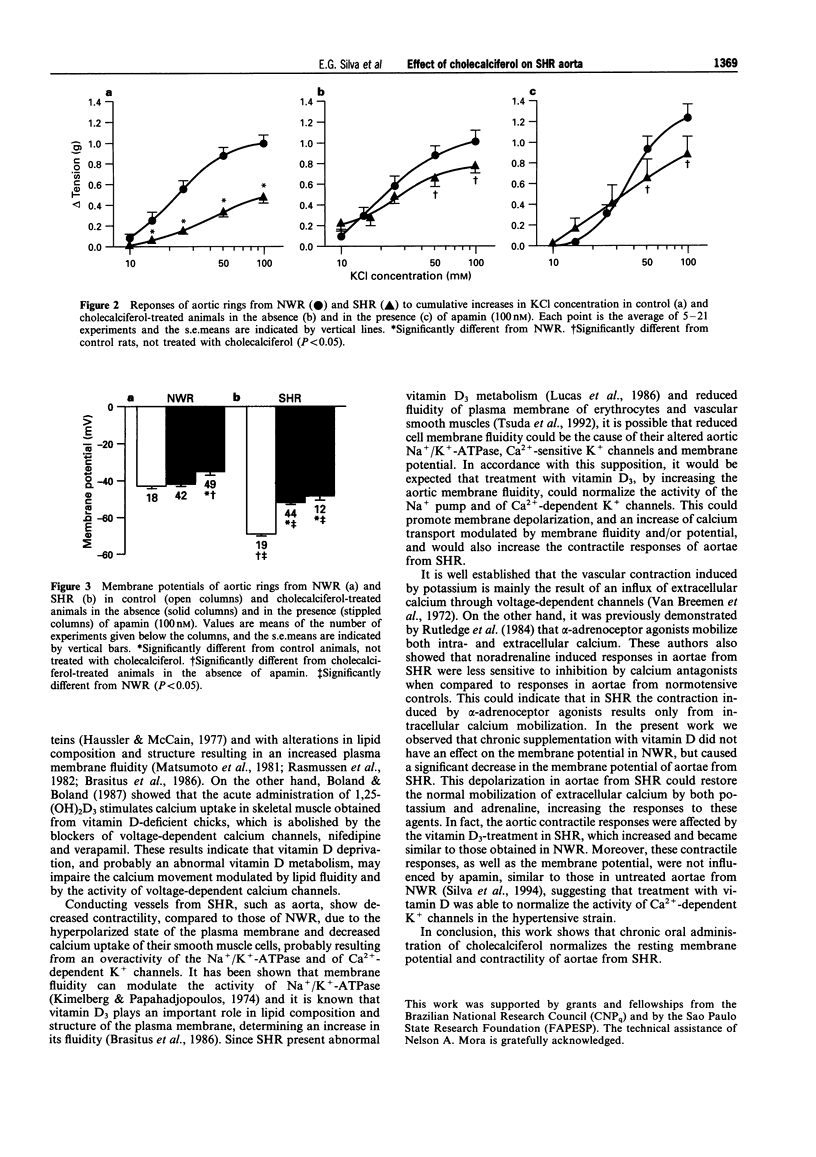
1. The diet of spontaneously hypertensive rats (SHR) and normotensive Wistar rats (NWR) was supplemented with 12.5 micrograms cholecalciferol per 100 g body weight daily, by gavage, for 4 weeks. 2. The amplitude of the contractile responses of aortic rings from SHR to potassium and adrenaline, which was smaller than in NWR aortae, was increased after treatment with cholecalciferol. No further changes were observed in the responses of NWR and SHR aortae in the presence of 100 nM apamin. 3. The membrane potentials of aortae from SHR, which were higher than those of aortae from NWR, decreased after treatment with cholecalciferol. Further depolarization was observed in aortic rings from NWR, but not in aortic rings from SHR, after their preincubation with 100 nM apamin. 4. It is concluded that cholecalciferol normalizes the membrane potential and contractility of aortae from SHR, probably through an effect on lipid composition and structure of the plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel M. B., Sharma R. V., Bhalla R. C. Increased 45Ca influx in response to alpha 1-adrenoceptor stimulation in spontaneously hypertensive rat caudal artery. J Cardiovasc Pharmacol. 1987 Aug;10(2):205–212. doi: 10.1097/00005344-198708000-00011. [DOI] [PubMed] [Google Scholar]
- Bohr D. F., Webb R. C. Vascular smooth muscle membrane in hypertension. Annu Rev Pharmacol Toxicol. 1988;28:389–409. doi: 10.1146/annurev.pa.28.040188.002133. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Dudeja P. K., Eby B., Lau K. Correction by 1-25-dihydroxycholecalciferol of the abnormal fluidity and lipid composition of enterocyte brush border membranes in vitamin D-deprived rats. J Biol Chem. 1986 Dec 15;261(35):16404–16409. [PubMed] [Google Scholar]
- Bukoski R. D., Xue H., McCarron D. A. Effect of 1,25(OH)2 vitamin D3 and ionized Ca2+ on 45Ca uptake by primary cultures of aortic myocytes of spontaneously hypertensive and Wistar Kyoto normotensive rats. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1330–1335. doi: 10.1016/0006-291x(87)90795-9. [DOI] [PubMed] [Google Scholar]
- Frediani-Neto E., Silva E. G., Paiva T. B., Paiva A. C. Increased intracellular Na+ and depolarization favor angiotension tachyphylaxis in rabbit aorta. Am J Physiol. 1991 Feb;260(2 Pt 2):H373–H378. doi: 10.1152/ajpheart.1991.260.2.H373. [DOI] [PubMed] [Google Scholar]
- Hallbäck M., Lundgren Y., Weiss L. Reactivity to noradrenaline of aortic strips and portal veins from spontaneously hypertensive and normotensive rats. Acta Physiol Scand. 1971 Feb;81(2):176–181. doi: 10.1111/j.1748-1716.1971.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Hatton D. C., Xue H., DeMerritt J. A., McCarron D. A. 1,25(OH)2 vitamin D3-induced alterations in vascular reactivity in the spontaneously hypertensive rat. Am J Med Sci. 1994 Feb;307 (Suppl 1):S154–S158. [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (second of two parts). N Engl J Med. 1977 Nov 10;297(19):1041–1050. doi: 10.1056/NEJM197711102971906. [DOI] [PubMed] [Google Scholar]
- Huzoor-Akbar, Chen N. Y., Fossen D. V., Wallace D. Increased vascular contractile sensitivity to serotonin in spontaneously hypertensive rats is linked with increased turnover of phosphoinositide. Life Sci. 1989;45(7):577–583. doi: 10.1016/0024-3205(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Lucas P. A., Brown R. C., Drüeke T., Lacour B., Metz J. A., McCarron D. A. Abnormal vitamin D metabolism, intestinal calcium transport, and bone calcium status in the spontaneously hypertensive rat compared with its genetic control. J Clin Invest. 1986 Jul;78(1):221–227. doi: 10.1172/JCI112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- NICOLAYSEN R., EEG-LARSEN N., MALM O. J. Physiology of calcium metabolism. Physiol Rev. 1953 Jul;33(3):424–444. doi: 10.1152/physrev.1953.33.3.424. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Matsumoto T., Fontaine O., Goodman D. B. Role of changes in membrane lipid structure in the action of 1,25-dihydroxyvitamin D3. Fed Proc. 1982 Jan;41(1):72–77. [PubMed] [Google Scholar]
- Rutledge A., Swamy V. C., Triggle D. J. Calcium-dependence and antagonism of responses to alpha 1- and alpha 2-adrenoceptor agonists in vascular tissues from hypertensive and normotensive rats. Br J Pharmacol. 1984 Sep;83(1):103–111. doi: 10.1111/j.1476-5381.1984.tb10124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Kurahashi K., Kuchii M. A possible etiology of contractility impairment of vascular smooth muscle from spontaneously hypertensive rats. J Pharmacol Exp Ther. 1973 May;185(2):406–417. [PubMed] [Google Scholar]
- Silva E. G., Frediani-Neto E., Ferreira A. T., Paiva A. C., Paiva T. B. Role of Ca(+)-dependent K-channels in the membrane potential and contractility of aorta from spontaneously hypertensive rats. Br J Pharmacol. 1994 Nov;113(3):1022–1028. doi: 10.1111/j.1476-5381.1994.tb17095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle C. R., Laher I. A review of changes in vascular smooth muscle functions in hypertension: isolated tissue versus in vivo studies. Can J Physiol Pharmacol. 1985 Apr;63(4):355–365. doi: 10.1139/y85-065. [DOI] [PubMed] [Google Scholar]
- Tsuda K., Ueno Y., Nishio I., Masuyama Y. Membrane fluidity as a genetic marker of hypertension. Clin Exp Pharmacol Physiol Suppl. 1992;20:11–16. [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Xue H., McCarron D. A., Bukoski R. D. 1,25 (OH)2 vitamin D3-induced 45CA uptake in vascular myocytes cultured from spontaneously hypertensive and normotensive rats. Life Sci. 1991;49(9):651–659. doi: 10.1016/0024-3205(91)90111-n. [DOI] [PubMed] [Google Scholar]
- de Boland A. R., Boland R. L. Rapid changes in skeletal muscle calcium uptake induced in vitro by 1,25-dihydroxyvitamin D3 are suppressed by calcium channel blockers. Endocrinology. 1987 May;120(5):1858–1864. doi: 10.1210/endo-120-5-1858. [DOI] [PubMed] [Google Scholar]



