Abstract
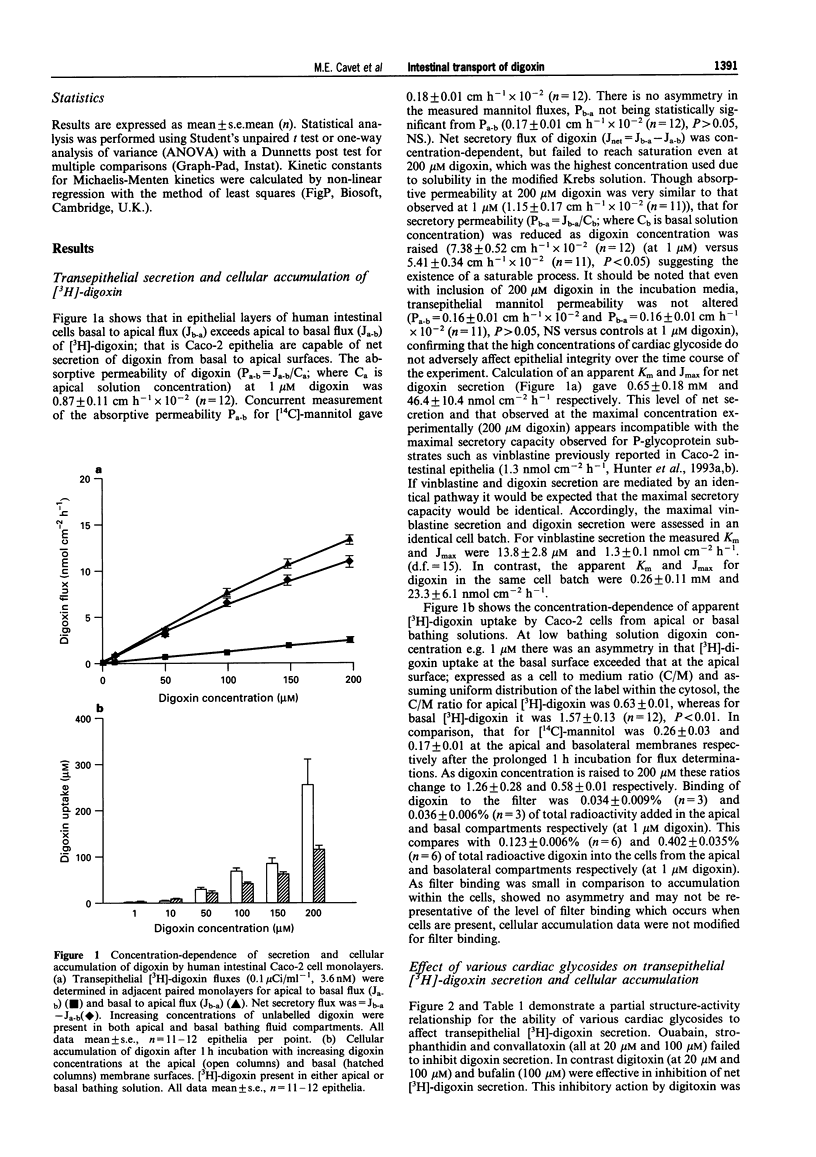
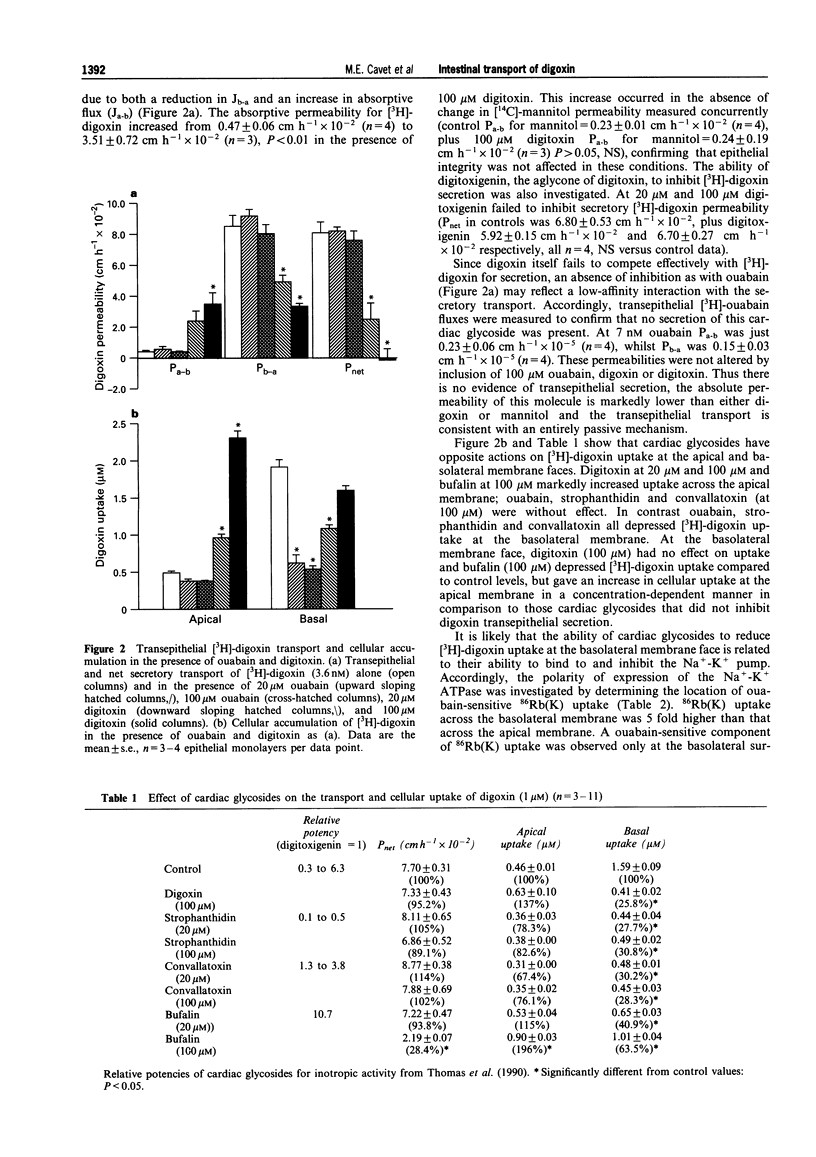
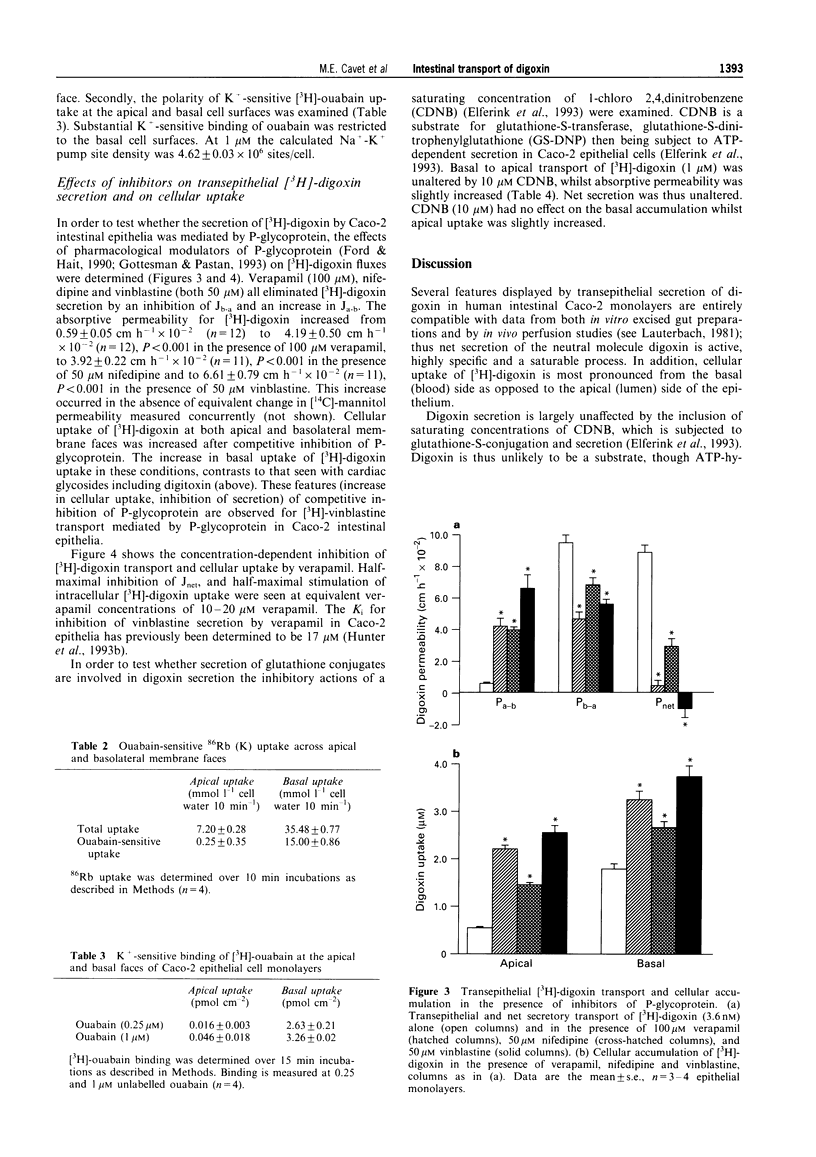
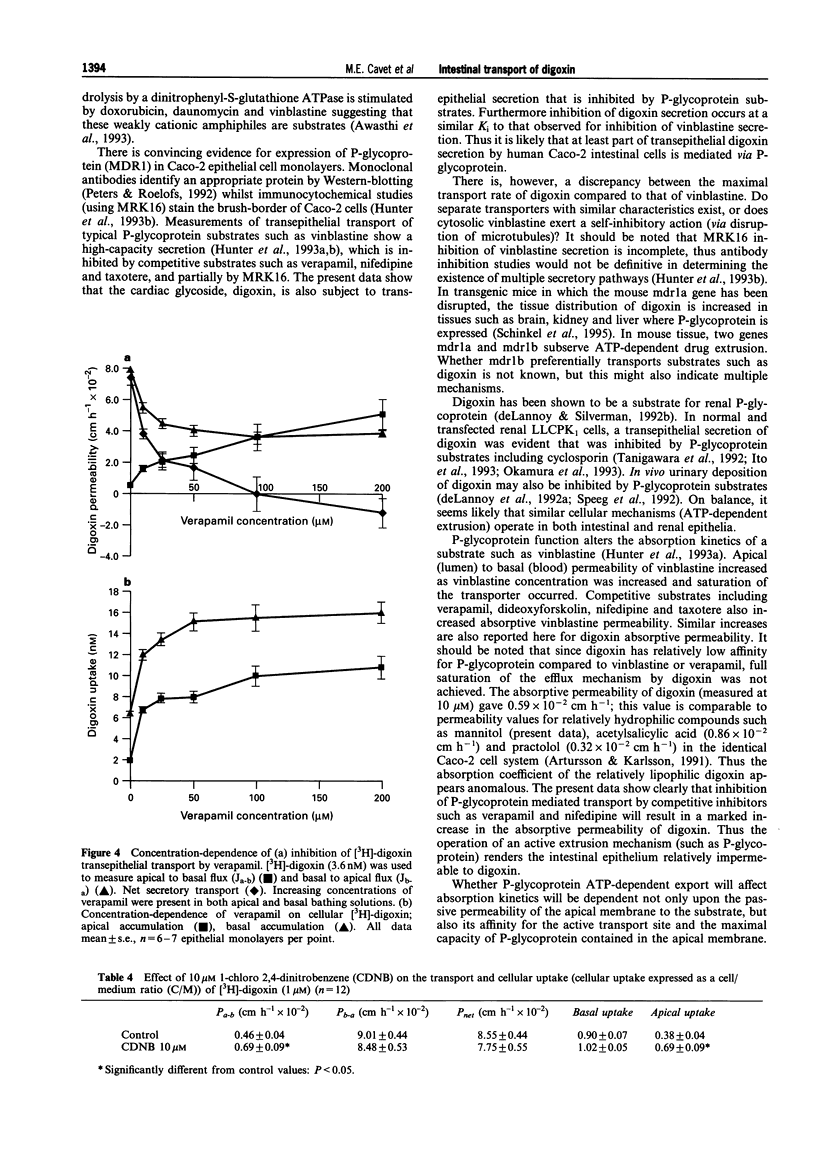
1. Human intestinal epithelial Caco-2 cells have been used to investigate the transepithelial permeation of the cardiac glycoside, digoxin. 2. Transepithelial basal to apical [3H]-digoxin flux exceeds apical to basal flux, a net secretion of [3H]-digoxin being observed. At 200 microM digoxin, net secretory flux (Jnet) was 10.8 +/- 0.6 nmol cm-2 h-1. Maximal secretory flux (Jmax) of vinblastine was 1.3 +/- 0.1 nmol cm-2 h-1. Cellular uptake of digoxin was different across apical and basal cell boundaries. It was greatest across the basal surface at 1 microM, whereas at 200 microM, apical uptake exceeded basal uptake. 3. Net secretion of [3H]-digoxin was subject to inhibition by digitoxin and bufalin but was not inhibited by ouabain, convallatoxin, and strophanthidin (all 100 microM). Inhibition was due to both a decrease in Jb-a and an increase in Ja-b. Uptake of [3H]-digoxin at the apical surface was increased by digitoxin and bufalin. All cardiac glycosides decreased [3H]-digoxin uptake at the basal cell surface (except for 100 microM digitoxin). 4. The competitive P-glycoprotein inhibitors, verapamil (100 microM), nifedipine (50 microM) and vinblastine (50 microM) all abolished net secretion of [3H]-digoxin due to both a decrease in Jb-a and an increase in Ja-b. Cellular accumulation of [3H]-digoxin was also increased across both the apical and basal cell surfaces. I-Chloro-2,4,-dinitrobenzene (10 microM), a substrate for glutathione-S-transferase and subsequent ATP-dependent glutathione-S-conjugate secretion, failed to inhibit net secretion of [3H]-digoxin. The increase in absorptive permeability Pa-b (= Ja-b/Ca) and cellular [3H]-digoxin uptake upon P-glycoprotein inhibition, showed that the intestinal epithelium was rendered effectively impermeable by ATP-dependent extrusion at the apical surface. 5. A model for [3H]-digoxin secretion by the intestinal epithelium is likely to involve both diffusional uptake and Na(+)-K+ pump-mediated endocytosis, followed by active extrusion at the apical membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Brown C. D., Ogden P., Simmons N. L. K+ transport in "tight' epithelial monolayers of MDCK cells. J Membr Biol. 1982;65(1-2):99–109. doi: 10.1007/BF01870473. [DOI] [PubMed] [Google Scholar]
- Artursson P., Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991 Mar 29;175(3):880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Singhal S. S., Srivastava S. K., Zimniak P., Bajpai K. K., Saxena M., Sharma R., Ziller S. A., 3rd, Frenkel E. P., Singh S. V. Adenosine triphosphate-dependent transport of doxorubicin, daunomycin, and vinblastine in human tissues by a mechanism distinct from the P-glycoprotein. J Clin Invest. 1994 Mar;93(3):958–965. doi: 10.1172/JCI117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. S., Conradi R. A., Hilgers A. R., Ho N. F. Evidence for a polarized efflux system for peptides in the apical membrane of Caco-2 cells. Biochem Biophys Res Commun. 1993 Feb 15;190(3):760–766. doi: 10.1006/bbrc.1993.1114. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Damm K. H., Woermann C. The effect of probenecid on the in vitro absorption of cardiac glycosides. Eur J Pharmacol. 1974 Sep;28(1):157–163. doi: 10.1016/0014-2999(74)90127-7. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H., Bergin L. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim Biophys Acta. 1990 Sep 7;1027(3):211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- De Lannoy I. A., Koren G., Klein J., Charuk J., Silverman M. Cyclosporin and quinidine inhibition of renal digoxin excretion: evidence for luminal secretion of digoxin. Am J Physiol. 1992 Oct;263(4 Pt 2):F613–F622. doi: 10.1152/ajprenal.1992.263.4.F613. [DOI] [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Hunter J., Jepson M. A., Tsuruo T., Simmons N. L., Hirst B. H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J Biol Chem. 1993 Jul 15;268(20):14991–14997. [PubMed] [Google Scholar]
- Inui K., Yamamoto M., Saito H. Transepithelial transport of oral cephalosporins by monolayers of intestinal epithelial cell line Caco-2: specific transport systems in apical and basolateral membranes. J Pharmacol Exp Ther. 1992 Apr;261(1):195–201. [PubMed] [Google Scholar]
- Ito S., Koren G., Harper P. A., Silverman M. Energy-dependent transport of digoxin across renal tubular cell monolayers (LLC-PK1). Can J Physiol Pharmacol. 1993 Jan;71(1):40–47. doi: 10.1139/y93-006. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Kuo S. M., Ziemniak J., Artursson P. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. Br J Pharmacol. 1993 Nov;110(3):1009–1016. doi: 10.1111/j.1476-5381.1993.tb13914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P. Internalization of ouabain and replacement of sodium pumps in the plasma membranes of HeLa cells following block with cardiac glycosides. Q J Exp Physiol. 1982 Jan;67(1):105–119. doi: 10.1113/expphysiol.1982.sp002605. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P., Simmons N. L. Autoradiographic localisation of [3H]ouabain bound to cultured epithelial cell monolayers of MDCK cells. Biochim Biophys Acta. 1981 Jun 22;644(2):333–340. doi: 10.1016/0005-2736(81)90391-6. [DOI] [PubMed] [Google Scholar]
- Okamura N., Hirai M., Tanigawara Y., Tanaka K., Yasuhara M., Ueda K., Komano T., Hori R. Digoxin-cyclosporin A interaction: modulation of the multidrug transporter P-glycoprotein in the kidney. J Pharmacol Exp Ther. 1993 Sep;266(3):1614–1619. [PubMed] [Google Scholar]
- Oude Elferink R. P., Bakker C. T., Jansen P. L. Glutathione-conjugate transport by human colon adenocarcinoma cells (Caco-2 cells). Biochem J. 1993 Mar 15;290(Pt 3):759–764. doi: 10.1042/bj2900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. H., Roelofs H. M. Biochemical characterization of resistance to mitoxantrone and adriamycin in Caco-2 human colon adenocarcinoma cells: a possible role for glutathione S-transferases. Cancer Res. 1992 Apr 1;52(7):1886–1890. [PubMed] [Google Scholar]
- Schinkel A. H., Wagenaar E., van Deemter L., Mol C. A., Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995 Oct;96(4):1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeg K. V., Maldonado A. L., Liaci J., Muirhead D. Effect of cyclosporine on colchicine secretion by the kidney multidrug transporter studied in vivo. J Pharmacol Exp Ther. 1992 Apr;261(1):50–55. [PubMed] [Google Scholar]
- Tanigawara Y., Okamura N., Hirai M., Yasuhara M., Ueda K., Kioka N., Komano T., Hori R. Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J Pharmacol Exp Ther. 1992 Nov;263(2):840–845. [PubMed] [Google Scholar]
- Thwaites D. T., Brown C. D., Hirst B. H., Simmons N. L. H(+)-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim Biophys Acta. 1993 Sep 19;1151(2):237–245. doi: 10.1016/0005-2736(93)90108-c. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., Brown C. D., Hirst B. H., Simmons N. L. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H(+)-coupled carriers at both apical and basal membranes. J Biol Chem. 1993 Apr 15;268(11):7640–7642. [PubMed] [Google Scholar]



