Abstract
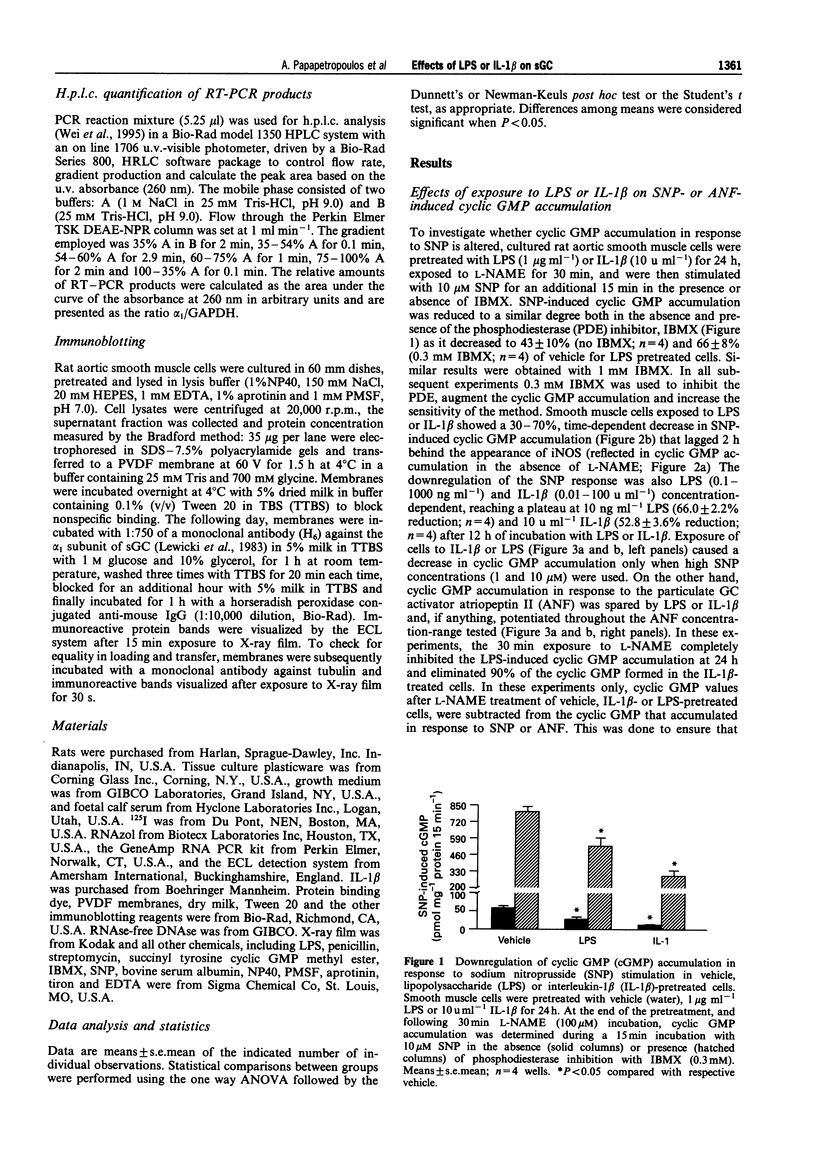
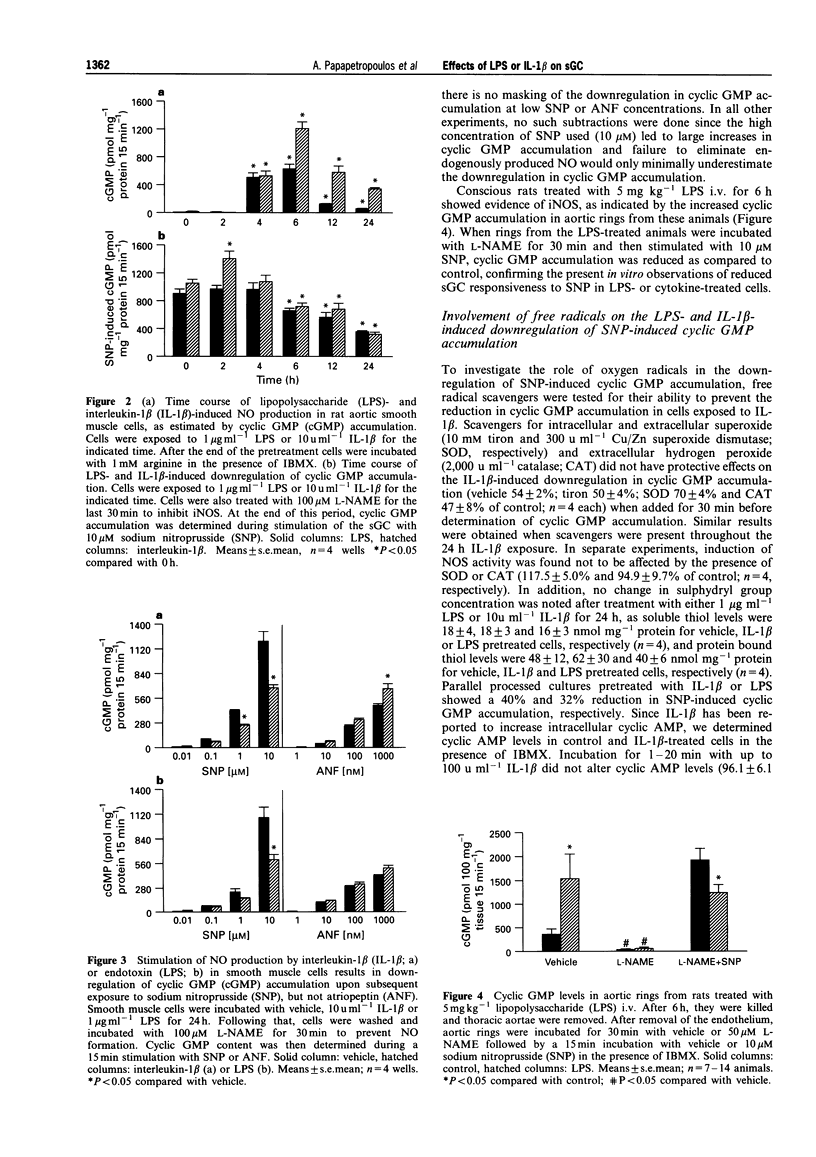
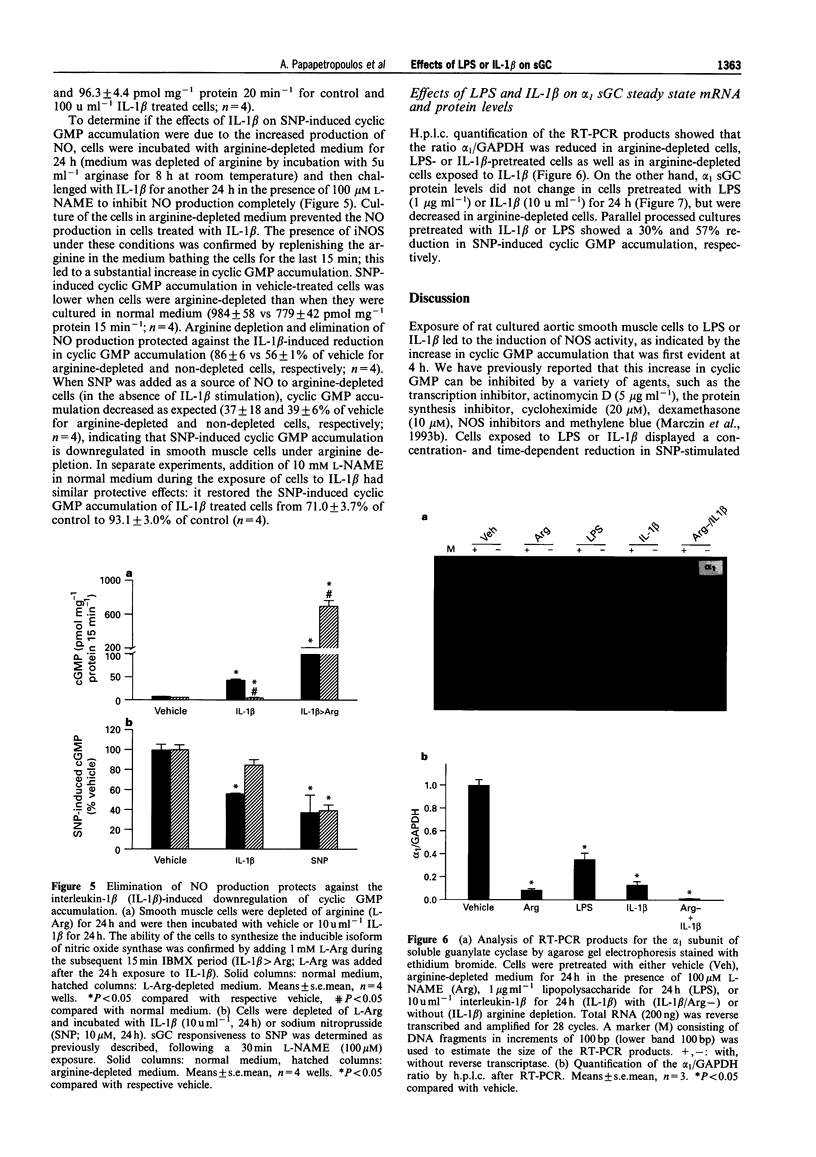
1. Induction of nitric oxide synthase (iNOS) results in overproduction of nitric oxide (NO), which may be a principal cause of the massive vasodilatation and hypotension observed in septic shock. Since NO-induced vasorelaxation is mediated via the soluble isoform of guanylate cyclase (sGC), the regulation of sGC activity during shock is of obvious importance, but yet poorly understood. The aim of the present study was to investigate the activation of sGC by sodium nitroprusside (SNP) before and after exposure of rat aortic smooth muscle cells to endotoxin (LPS) or interleukin-1 beta (IL-1 beta). 2. Exposure of rat aortic smooth muscle cells to SNP (10 microM) elicited up to 200 fold increases in cyclic GMP. This effect was attenuated by 30-70% in IL-1 beta- or LPS-pretreated cells, in a pretreatment time-and IL-1 beta- or LPS-concentration-dependent manner. When, however, cells were exposed to IL-1 beta or LPS and then stimulated with the particulate guanylate cyclase activator, atriopeptin II, no reduction in cyclic GMP accumulation was observed. 3. Pretreatment of rats with LPS (5 mg kg-1, i.v.) for 6 h led to a decrease in aortic ring SNP-induced cyclic GMP accumulation. 4. The IL-1 beta-induced reduction in SNP-stimulated cyclic GMP accumulation in cultured cells was dependent on NO production, as arginine depletion abolished the downregulation of cyclic GMP accumulation in response to SNP. 5. Reverse-transcriptase-polymerase chain reaction analysis revealed that the ratio of steady state mRNA for the alpha, subunit of sGC to glyceraldehyde phosphate dehydrogenase was decreased in LPS- or IL-1 beta-treated cells, as compared to vehicle-treated cells. 6. Protein levels of the alpha 1 sGC subunit remained unaltered upon exposure to LPS or IL-1 beta, suggesting that the early decreased cyclic GMP accumulation in IL-1 beta- or LPS-pretreated cells was probably due to reduced sGC activation. Thus, the observed decreased responsiveness of sGC to NO stimulation following cytokine or LPS challenge may represent an important homeostatic mechanism to offset the extensive vasodilatation seen in sepsis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandwein H. J., Lewicki J. A., Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem. 1981 Mar 25;256(6):2958–2962. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Buechler W. A., Nakane M., Murad F. Expression of soluble guanylate cyclase activity requires both enzyme subunits. Biochem Biophys Res Commun. 1991 Jan 15;174(1):351–357. doi: 10.1016/0006-291x(91)90527-e. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- De Kimpe S. J., Van Heuven-Nolsen D., van Amsterdam J. G., Radomski M. W., Nijkamp F. P. Induction of nitric oxide release by interferon-gamma inhibits vasodilation and cyclic GMP increase in bovine isolated mesenteric arteries. J Pharmacol Exp Ther. 1994 Feb;268(2):910–915. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Evans T., Carpenter A., Cohen J. Inducible nitric-oxide-synthase mRNA is transiently expressed and destroyed by a cycloheximide-sensitive process. Eur J Biochem. 1994 Jan 15;219(1-2):563–569. doi: 10.1111/j.1432-1033.1994.tb19972.x. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Geisel J., Cook J. A., Coffee K. A., Wise W. C., Halushka P. V. Endotoxin-induced arachidonic acid metabolism requires de novo protein synthesis and protein kinase C activation. Biochim Biophys Acta. 1991 Aug 20;1085(1):15–20. doi: 10.1016/0005-2760(91)90226-8. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Gierse J. K., Hauser S. D., Creely D. P., Koboldt C., Rangwala S. H., Isakson P. C., Seibert K. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J. 1995 Jan 15;305(Pt 2):479–484. doi: 10.1042/bj3050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscavage J. M., Rogers N. E., Sherman M. P., Ignarro L. J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993 Dec 1;151(11):6329–6337. [PubMed] [Google Scholar]
- Henry P. J., Drummer O. H., Horowitz J. D. S-nitrosothiols as vasodilators: implications regarding tolerance to nitric oxide-containing vasodilators. Br J Pharmacol. 1989 Nov;98(3):757–766. doi: 10.1111/j.1476-5381.1989.tb14603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojda G., Beck J. K., Meyer W., Noack E. Nitrovasodilator-induced relaxation and tolerance development in porcine vena cordis magna: dependence on intact endothelium. Br J Pharmacol. 1994 Jun;112(2):533–540. doi: 10.1111/j.1476-5381.1994.tb13106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., McQuillan L. P., Leung G. K., Faller D. V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993 Jul;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki J. A., Chang B., Murad F. Quantification of guanylate cyclase concentrations by a direct double determinant tandem immunoradiometric assay. J Biol Chem. 1983 Mar 25;258(6):3509–3515. [PubMed] [Google Scholar]
- Magrinat G., Mason S. N., Shami P. J., Weinberg J. B. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992 Oct 15;80(8):1880–1884. [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Jilling T., Catravas J. D. Prevention of nitric oxide synthase induction in vascular smooth muscle cells by microtubule depolymerizing agents. Br J Pharmacol. 1993 Jul;109(3):603–605. doi: 10.1111/j.1476-5381.1993.tb13613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A., Go C. Y., Murad F., Catravas J. D. Mechanisms of tolerance to sodium nitroprusside in rat cultured aortic smooth muscle cells. Br J Pharmacol. 1996 Jan;117(1):147–155. doi: 10.1111/j.1476-5381.1996.tb15167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A., Marczin N., Mora G., Milici A., Murad F., Catravas J. D. Regulation of vascular smooth muscle soluble guanylate cyclase activity, mRNA, and protein levels by cAMP-elevating agents. Hypertension. 1995 Oct;26(4):696–704. doi: 10.1161/01.hyp.26.4.696. [DOI] [PubMed] [Google Scholar]
- Potter L. R., Garbers D. L. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J Biol Chem. 1994 May 20;269(20):14636–14642. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shimouchi A., Janssens S. P., Bloch D. B., Zapol W. M., Bloch K. D. cAMP regulates soluble guanylate cyclase beta 1-subunit gene expression in RFL-6 rat fetal lung fibroblasts. Am J Physiol. 1993 Nov;265(5 Pt 1):L456–L461. doi: 10.1152/ajplung.1993.265.5.L456. [DOI] [PubMed] [Google Scholar]
- Tsuchida S., Hiraoka M., Sudo M., Kigoshi S., Muramatsu I. Attenuation of sodium nitroprusside responses after prolonged incubation of rat aorta with endotoxin. Am J Physiol. 1994 Dec;267(6 Pt 2):H2305–H2310. doi: 10.1152/ajpheart.1994.267.6.H2305. [DOI] [PubMed] [Google Scholar]
- Ujiie K., Drewett J. G., Yuen P. S., Star R. A. Differential expression of mRNA for guanylyl cyclase-linked endothelium-derived relaxing factor receptor subunits in rat kidney. J Clin Invest. 1993 Feb;91(2):730–734. doi: 10.1172/JCI116255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Wei J., Milici A., Buccafusco J. J. Alterations in the expression of the genes encoding specific muscarinic receptor subtypes in the hypothalamus of spontaneously hypertensive rats. Circ Res. 1995 Jan;76(1):142–147. doi: 10.1161/01.res.76.1.142. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]