Abstract
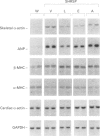
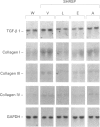
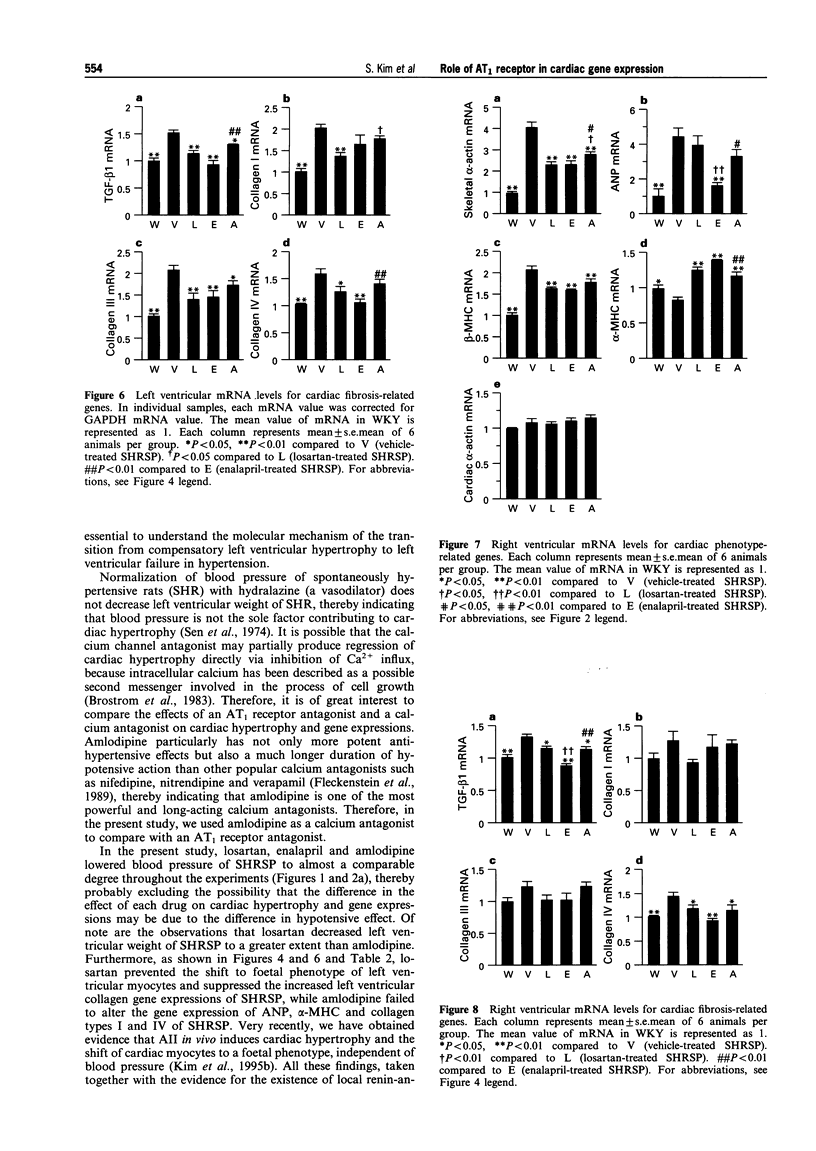
1. This study was undertaken to determine whether the AT1 receptor directly contributes to hypertension-induced cardiac hypertrophy and gene expressions. 2. Stroke-prone spontaneously hypertensive rats (SHRSP) were given orally an AT1, receptor antagonist (losartan, 30 mg kg-1 day-1), an angiotensin converting enzyme inhibitor (enalapril 10 mg kg-1 day-1), a dihydropyridine calcium channel antagonist (amlodipine, 5 mg kg-1 day-1), or vehicle (control), for 8 weeks (from 16 to 24 weeks of age). The effects of each drug were compared on ventricular weight and mRNA levels for myocardial phenotype- and fibrosis-related genes. 3. Left ventricular hypertrophy of SHRSP was accompanied by the increase in mRNA levels for two foetal phenotypes of contractile proteins (skeletal alpha-actin and beta-myosin heavy chain (beta-MHC)), atrial natriuretic polypeptide (ANP), transforming growth factor-beta-1 (TGF-beta 1) and collagen, and a decrease in mRNA levels for an adult phenotype of contractile protein (alpha-MHC). Thus, the left ventricle of SHRSP was characterized by myocardial transition from an adult to a foetal phenotype and interstitial fibrosis at the molecular level. 4. Although losartan, enalapril and amlodipine lowered blood pressure of SHRSP to a comparable degree throughout the treatment, losartan caused regression of left ventricular hypertrophy of SHRSP to a greater extent than amlodipine (P < 0.01). 5. Losartan significantly decreased mRNA levels for skeletal alpha-actin, ANP, TGF-beta 1 and collagen types I, III and IV and increased alpha-MHC mRNA in the left ventricle of SHRSP. Amlodipine did not alter left ventricular ANP, alpha-MHC and collagen types I and IV mRNA levels of SHRSP. 6. The effects of enalapril on left ventricular hypertrophy and gene expressions of SHRSP were similar to those of losartan, except for the lack of inhibition of collagen type I expression by enalapril. 7. Unlike the hypertrophied left ventricle, there was no significant difference between losartan and amlodipine in the effects on non-hypertrophied right ventricular gene expressions of SHRSP. 8. Our results show that hypertension causes not only left ventricular hypertrophy but also molecular transition of myocardium to a foetal phenotype and interstitial fibrosis-related molecular changes. These hypertension-induced left ventricular molecular changes may be at least in part mediated by the direct action of local angiotensin II via the AT1, receptor.
Full text
PDF

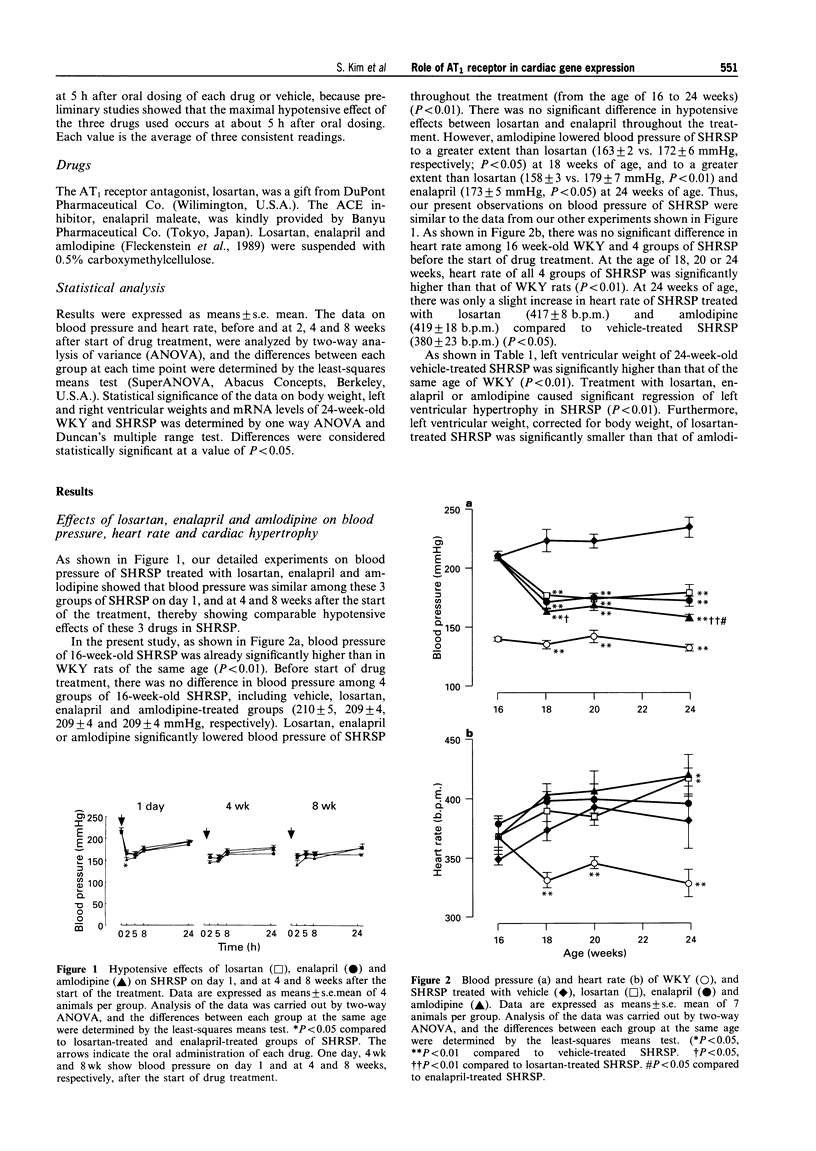

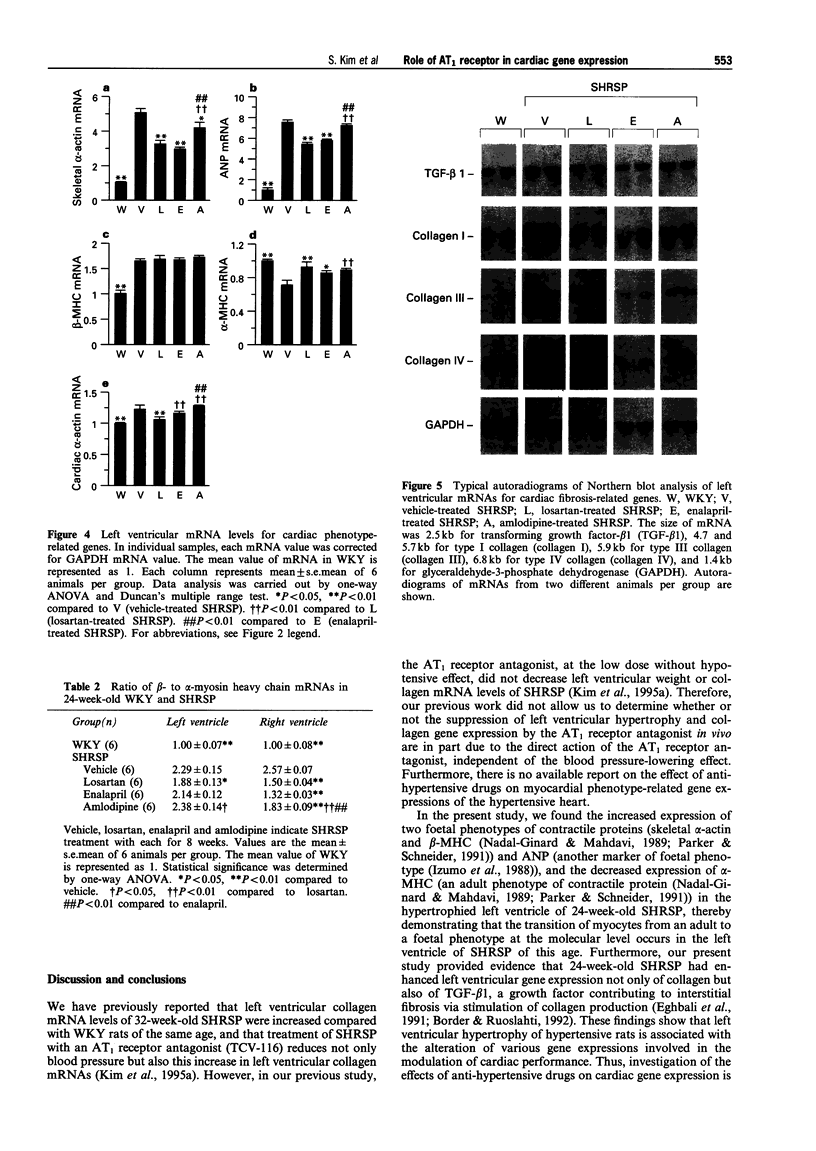


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983 Dec 10;258(23):14390–14399. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doering C. W., Jalil J. E., Janicki J. S., Pick R., Aghili S., Abrahams C., Weber K. T. Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res. 1988 Oct;22(10):686–695. doi: 10.1093/cvr/22.10.686. [DOI] [PubMed] [Google Scholar]
- Dorn G. W., 2nd, Robbins J., Ball N., Walsh R. A. Myosin heavy chain regulation and myocyte contractile depression after LV hypertrophy in aortic-banded mice. Am J Physiol. 1994 Jul;267(1 Pt 2):H400–H405. doi: 10.1152/ajpheart.1994.267.1.H400. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Tomek R., Sukhatme V. P., Woods C., Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res. 1991 Aug;69(2):483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Morkin E. Analysis of thyroid hormone effects on myosin heavy chain gene expression in cardiac and soleus muscles using a novel dot-blot mRNA assay. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1161–1167. doi: 10.1016/0006-291x(85)91737-1. [DOI] [PubMed] [Google Scholar]
- Hewett T. E., Grupp I. L., Grupp G., Robbins J. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res. 1994 Apr;74(4):740–746. doi: 10.1161/01.res.74.4.740. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B., Levy D., Cupples L. A. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol. 1987;10 (Suppl 6):S135–S140. [PubMed] [Google Scholar]
- Kim S., Ohta K., Hamaguchi A., Omura T., Yukimura T., Miura K., Inada Y., Ishimura Y., Chatani F., Iwao H. Angiotensin II type I receptor antagonist inhibits the gene expression of transforming growth factor-beta 1 and extracellular matrix in cardiac and vascular tissues of hypertensive rats. J Pharmacol Exp Ther. 1995 Apr;273(1):509–515. [PubMed] [Google Scholar]
- Kim S., Ohta K., Hamaguchi A., Omura T., Yukimura T., Miura K., Inada Y., Wada T., Ishimura Y., Chatani F. Contribution of renal angiotensin II type I receptor to gene expressions in hypertension-induced renal injury. Kidney Int. 1994 Nov;46(5):1346–1358. doi: 10.1038/ki.1994.404. [DOI] [PubMed] [Google Scholar]
- Kim S., Ohta K., Hamaguchi A., Yukimura T., Miura K., Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension. 1995 Jun;25(6):1252–1259. doi: 10.1161/01.hyp.25.6.1252. [DOI] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Lindpaintner K., Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991 Apr;68(4):905–921. doi: 10.1161/01.res.68.4.905. [DOI] [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer Y., Czosnek H., Zeelon P. E., Yaffe D., Nudel U. Expression of the genes coding for the skeletal muscle and cardiac actions in the heart. Nucleic Acids Res. 1984 Jan 25;12(2):1087–1100. doi: 10.1093/nar/12.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B., Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989 Dec;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Ohkubo H., Hirose T., Inayama S., Nakanishi S. mRNA sequence for human cardiodilatin-atrial natriuretic factor precursor and regulation of precursor mRNA in rat atria. Nature. 1984 Aug 23;310(5979):699–701. doi: 10.1038/310699a0. [DOI] [PubMed] [Google Scholar]
- Oberbäumer I., Laurent M., Schwarz U., Sakurai Y., Yamada Y., Vogeli G., Voss T., Siebold B., Glanville R. W., Kühn K. Amino acid sequence of the non-collagenous globular domain (NC1) of the alpha 1(IV) chain of basement membrane collagen as derived from complementary DNA. Eur J Biochem. 1985 Mar 1;147(2):217–224. doi: 10.1111/j.1432-1033.1985.tb08739.x. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Schneider M. D. Growth factors, proto-oncogenes, and plasticity of the cardiac phenotype. Annu Rev Physiol. 1991;53:179–200. doi: 10.1146/annurev.ph.53.030191.001143. [DOI] [PubMed] [Google Scholar]
- Perreault C. L., Bing O. H., Brooks W. W., Ransil B. J., Morgan J. P. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ Res. 1990 Sep;67(3):707–712. doi: 10.1161/01.res.67.3.707. [DOI] [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Roberts A. B., Sporn M. B. cDNA cloning by PCR of rat transforming growth factor beta-1. Nucleic Acids Res. 1990 May 25;18(10):3059–3059. doi: 10.1093/nar/18.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau J. L., Paradis P., Shenasa H., Juneau C. Faster time to peak tension and velocity of shortening in right versus left ventricular trabeculae and papillary muscles of dogs. Circ Res. 1986 Nov;59(5):556–561. doi: 10.1161/01.res.59.5.556. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Xu Y., Slayter H. S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993 Dec 3;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Sen S., Tarazi R. C., Khairallah P. A., Bumpus F. M. Cardiac hypertrophy in spontaneously hypertensive rats. Circ Res. 1974 Nov;35(5):775–781. doi: 10.1161/01.res.35.5.775. [DOI] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata H., Healy B., Stewart R. W., Bumpus F. M., Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990 Apr;66(4):883–890. doi: 10.1161/01.res.66.4.883. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Brilla C. G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991 Jun;83(6):1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]