Abstract
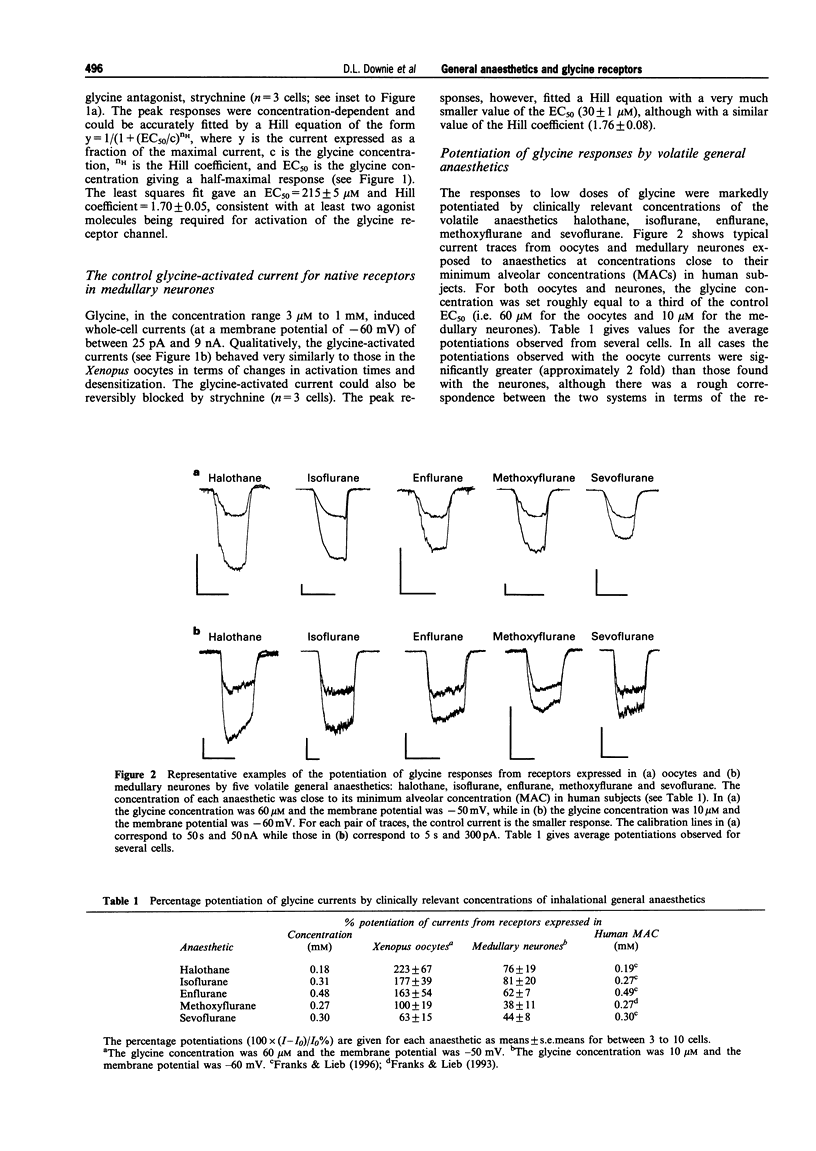
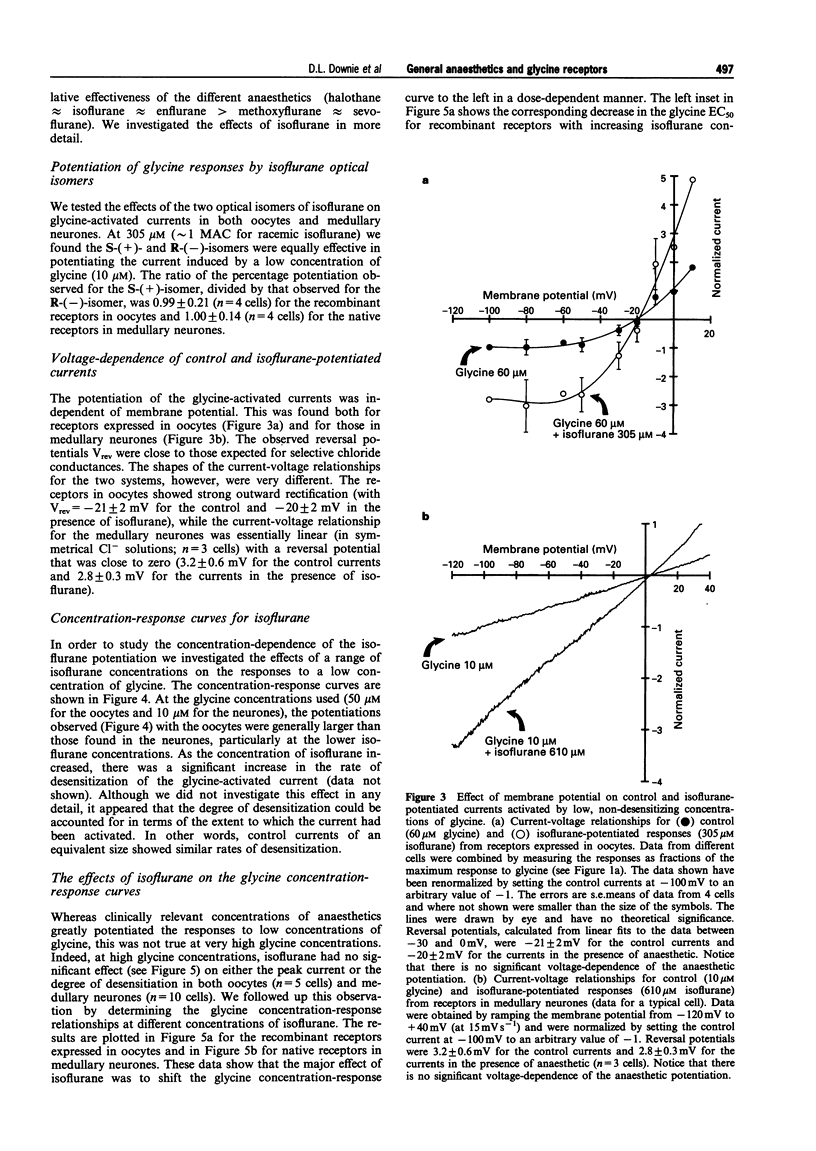
1. Glycine responses were studied under voltage clamp in Xenopus oocytes injected with cDNA encoding mammalian glycine receptor subunits and in rat medullary neurones. Bath application of glycine gave strychnine-sensitive currents which reversed close to the expected equilibrium potentials for chloride ions. The peak currents for the receptors expressed in oocytes fitted a Hill equation with EC50 = 215 +/- 5 microM and Hill coefficient nH = 1.70 +/- 0.05 (means +/- s.e. means). The peak currents from the receptors in medullary neurones fitted a Hill equation with EC50 = 30 +/- 1 microM and Hill coefficient nH = 1.76 +/- 0.08. The current-voltage relationship for the receptors expressed in oocytes showed strong outward rectification (with Vrev = -21 +/- 2 mV), while that for the glycine responses from the medullary neurones in symmetrical Cl- was linear (with Vrev = 3.2 +/- 0.6 mV). 2. Inhalational general anaesthetics, at concentrations close to their human minimum alveolar concentrations (MACs), potentiated responses to low concentrations of glycine. The potentiation observed with the recombinant receptors (between 60-22%) was approximately twice that found with the medullary neurones (between 40-80%). For both the recombinant receptors and the receptors in medullary neurones, the degree of potentiation increased in the order of methoxyflurane approximately sevoflurane < halothane approximately isoflurane approximately enflurane. There was no significant difference between the potentiations observed for the two optical isomers of isoflurane. 3. For both the recombinant and native receptors, isoflurane potentiated the currents in a dose-dependent manner at low concentrations of glycine, although at high glycine concentrations the anaesthetic had no significant effect on the glycine-activated responses. The major effect of isoflurane was to cause a parallel leftward shift in the glycine concentration-response curves. The glycine EC50 concentration for the recombinant receptors decreased from a control value of 215 +/- 5 microM to 84 +/- 7 microM glycine at 610 microM isoflurane, while that for the medullary neurones decreased from a control value of 30 +/- 1 microM to 18 +/- 2 microM glycine at the same concentration of isoflurane. The potentiation was independent of membrane potential. 4. Isoflurane also potentiated responses to taurine, a partial agonist at the glycine receptor. This was observed for receptors expressed in oocytes at both low and saturating concentrations of taurine. The EC50 concentration decreased from a control value of 1.6 +/- 0.2 to 0.9 +/- 0.1 mM taurine in the presence of 305 microM isoflurane, while the maximum response to taurine increased from 47 +/- 2 to 59 +/- 2% of the maximum response to glycine. 5. Glycine receptors, like other members of the fast ligand-gated receptor superfamily, are sensitive to clinically relevant concentrations of inhalational general anaesthetics. Effects at these receptors may, therefore, play some role in the maintenance of the anaesthetic state.
Full text
PDF

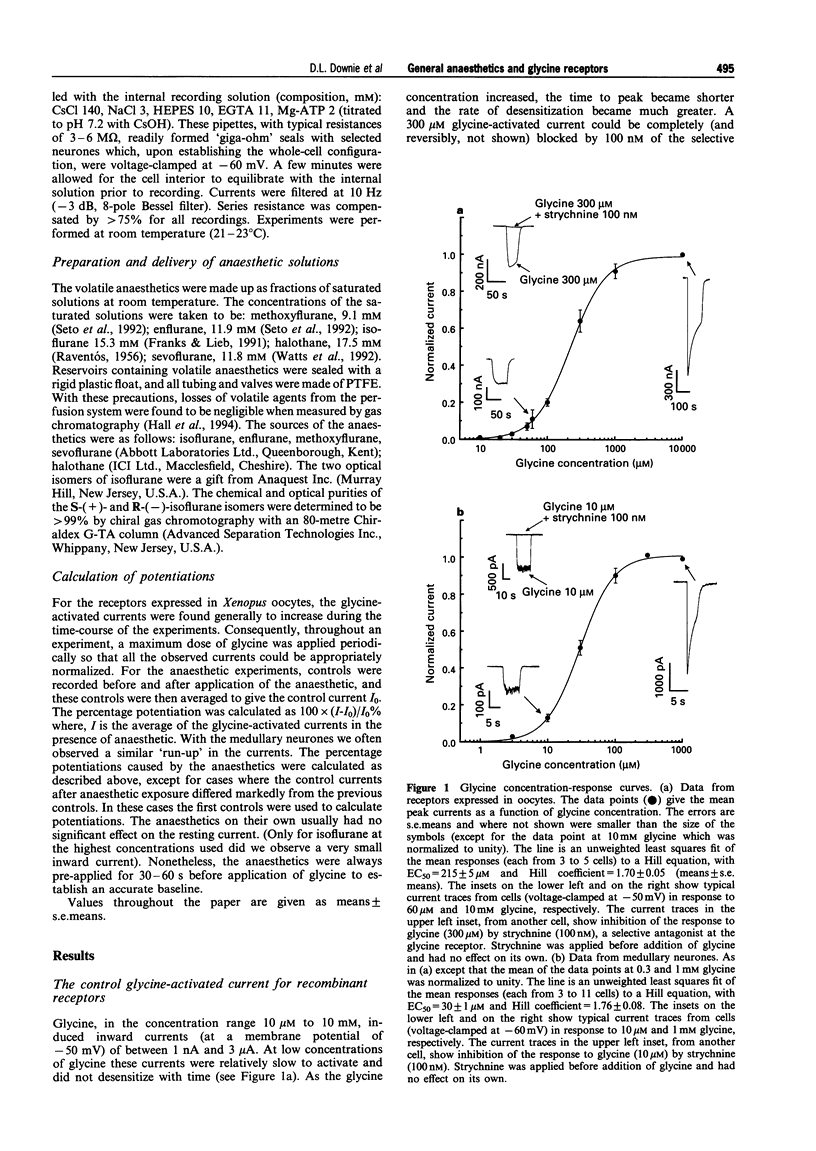
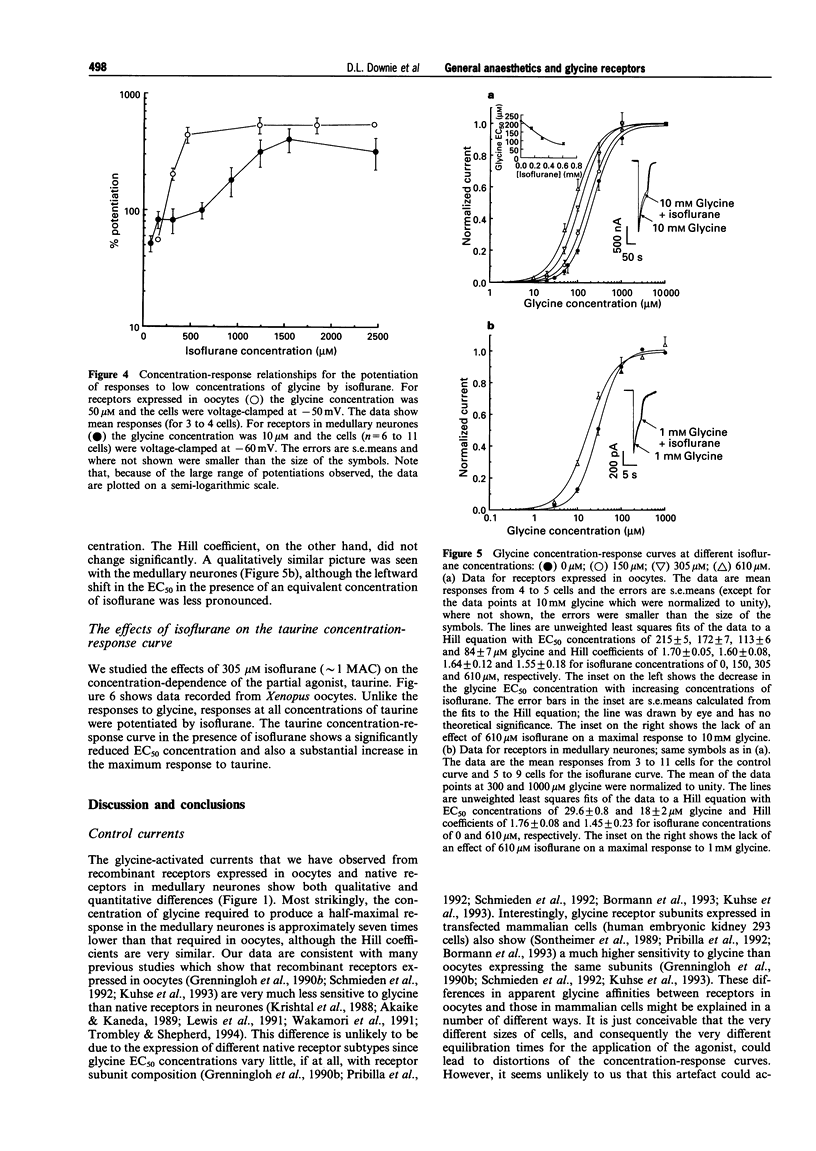



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Antognini J. F., Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993 Dec;79(6):1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- Arimura H., Ikemoto Y. Action of enflurane on cholinergic transmission in identified Aplysia neurones. Br J Pharmacol. 1986 Nov;89(3):573–582. doi: 10.1111/j.1476-5381.1986.tb11158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Structure and function of inhibitory glycine receptors. Q Rev Biophys. 1992 Nov;25(4):381–394. doi: 10.1017/s0033583500004340. [DOI] [PubMed] [Google Scholar]
- Bloomenthal A. B., Goldwater E., Pritchett D. B., Harrison N. L. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+. Mol Pharmacol. 1994 Dec;46(6):1156–1159. [PubMed] [Google Scholar]
- Bormann J., Rundström N., Betz H., Langosch D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993 Oct;12(10):3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S., Mody H. I. The effects of isoflurane on acetylcholine receptor channels.: 2. Currents elicited by rapid perfusion of acetylcholine. Mol Pharmacol. 1993 Nov;44(5):1056–1063. [PubMed] [Google Scholar]
- Downie D. L., Hope A. G., Belelli D., Lambert J. J., Peters J. A., Bentley K. R., Steward L. J., Chen C. Y., Barnes N. M. The interaction of trichloroethanol with murine recombinant 5-HT3 receptors. Br J Pharmacol. 1995 Apr;114(8):1641–1651. doi: 10.1111/j.1476-5381.1995.tb14952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone L. L., Sauter J. F., Braswell L. M., Miller K. W. Actions of general anesthetics on acetylcholine receptor-rich membranes from Torpedo californica. Anesthesiology. 1986 Jun;64(6):694–702. doi: 10.1097/00000542-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br J Anaesth. 1993 Jul;71(1):65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991 Oct 18;254(5030):427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology. 1996 Mar;84(3):716–720. doi: 10.1097/00000542-199603000-00027. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Properties of human brain glycine receptors expressed in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):235–244. doi: 10.1098/rspb.1984.0032. [DOI] [PubMed] [Google Scholar]
- Hales T. G., Lambert J. J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991 Nov;104(3):619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. C., Lieb W. R., Franks N. P. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br J Pharmacol. 1994 Jul;112(3):906–910. doi: 10.1111/j.1476-5381.1994.tb13166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris B., Moody E., Skolnick P. Isoflurane anesthesia is stereoselective. Eur J Pharmacol. 1992 Jul 7;217(2-3):215–216. doi: 10.1016/0014-2999(92)90875-5. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Kugler J. L., Jones M. V., Greenblatt E. P., Pritchett D. B. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993 Sep;44(3):628–632. [PubMed] [Google Scholar]
- Ito S., Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. V., Harrison N. L. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993 Oct;70(4):1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Osipchuk YuV, Vrublevsky S. V. Properties of glycine-activated conductances in rat brain neurones. Neurosci Lett. 1988 Feb 3;84(3):271–276. doi: 10.1016/0304-3940(88)90519-8. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Betz H., Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol. 1995 Jun;5(3):318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Laube B., Magalei D., Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993 Dec;11(6):1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990 Dec 25;265(36):22317–22320. [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A., Ahmed Z., Faber D. S. A characterization of glycinergic receptors present in cultured rat medullary neurons. J Neurophysiol. 1991 Oct;66(4):1291–1303. doi: 10.1152/jn.1991.66.4.1291. [DOI] [PubMed] [Google Scholar]
- Lysko G. S., Robinson J. L., Casto R., Ferrone R. A. The stereospecific effects of isoflurane isomers in vivo. Eur J Pharmacol. 1994 Sep 22;263(1-2):25–29. doi: 10.1016/0014-2999(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Machu T. K., Harris R. A. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994 Nov;271(2):898–905. [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D. S., Heath M. J., Gelber S., Devay P., Role L. W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995 Sep 22;269(5231):1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McKenzie D., Franks N. P., Lieb W. R. Actions of general anaesthetics on a neuronal nicotinic acetylcholine receptor in isolated identified neurones of Lymnaea stagnalis. Br J Pharmacol. 1995 May;115(2):275–282. doi: 10.1111/j.1476-5381.1995.tb15874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Moody E. J., Harris B. D., Skolnick P. Stereospecific actions of the inhalation anesthetic isoflurane at the GABAA receptor complex. Brain Res. 1993 Jun 25;615(1):101–106. doi: 10.1016/0006-8993(93)91119-d. [DOI] [PubMed] [Google Scholar]
- Morales A., Nguyen Q. T., Miledi R. Electrophysiological properties of newborn and adult rat spinal cord glycine receptors expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3097–3101. doi: 10.1073/pnas.91.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaki T., Ikeuchi Y. Activation of endogenous protein kinase C enhances currents through alpha 1 and alpha 2 glycine receptor channels. Brain Res. 1995 Jul 31;687(1-2):214–216. doi: 10.1016/0006-8993(95)00543-y. [DOI] [PubMed] [Google Scholar]
- Ortells M. O., Lunt G. G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995 Mar;18(3):121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992 Dec;11(12):4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan J. J., Firestone S., Firestone L. L. Isoflurane's enhancement of chloride flux through rat brain gamma-aminobutyric acid type A receptors is stereoselective. Anesthesiology. 1995 Sep;83(3):611–615. doi: 10.1097/00000542-199509000-00021. [DOI] [PubMed] [Google Scholar]
- RAVENTOS J. The action of fluothane; a new volatile anaesthetic. Br J Pharmacol Chemother. 1956 Dec;11(4):394–410. doi: 10.1111/j.1476-5381.1956.tb00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil I. J. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994 Mar;80(3):606–610. doi: 10.1097/00000542-199403000-00017. [DOI] [PubMed] [Google Scholar]
- Rampil I. J., Mason P., Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993 Apr;78(4):707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992 Jun;11(6):2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton C. J., Doyle M. G., Price D. J., Daniels S., Smith E. B. The effect of high pressure on glycine- and kainate-sensitive receptor channels expressed in Xenopus oocytes. Proc Biol Sci. 1993 Nov 22;254(1340):131–137. doi: 10.1098/rspb.1993.0137. [DOI] [PubMed] [Google Scholar]
- Siebler M., Pekel M., Köller H., Müller H. W. Strychnine-sensitive glycine receptors in cultured primary neurons from rat neocortex. Brain Res Dev Brain Res. 1993 Jun 8;73(2):289–292. doi: 10.1016/0165-3806(93)90149-5. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Colquhoun D. Acetylcholine receptors: too many channels, too few functions. Science. 1995 Sep 22;269(5231):1681–1682. doi: 10.1126/science.7569892. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Takagi T., Pribilla I., Kirsch J., Betz H. Coexpression of the receptor-associated protein gephyrin changes the ligand binding affinities of alpha 2 glycine receptors. FEBS Lett. 1992 Jun 1;303(2-3):178–180. doi: 10.1016/0014-5793(92)80513-g. [DOI] [PubMed] [Google Scholar]
- Taleb O., Betz H. Expression of the human glycine receptor alpha 1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 1994 Mar 15;13(6):1318–1324. doi: 10.1002/j.1460-2075.1994.tb06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley P. Q., Shepherd G. M. Glycine exerts potent inhibitory actions on mammalian olfactory bulb neurons. J Neurophysiol. 1994 Feb;71(2):761–767. doi: 10.1152/jn.1994.71.2.761. [DOI] [PubMed] [Google Scholar]
- Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 1993 Jan;72 (Suppl):31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Wakamori M., Ikemoto Y., Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991 Dec;66(6):2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Watts M. T., Escarzaga M., Williams C. H. Gas chromatographic headspace analysis of sevoflurane in blood. J Chromatogr. 1992 Jun 10;577(2):289–298. doi: 10.1016/0378-4347(92)80250-t. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]


