Abstract
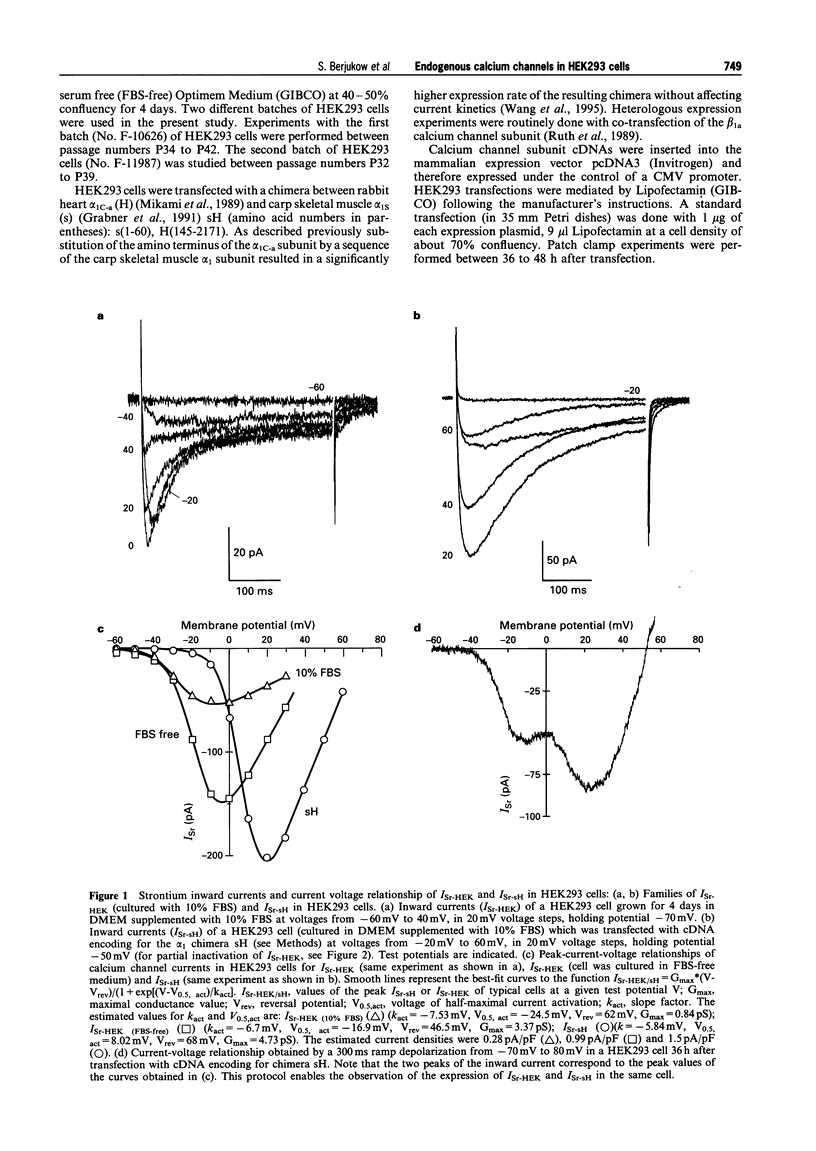
1. We have identified endogenous calcium channel currents in HEK293 cells. Whole cell endogenous currents (ISr-HEK) were studied in single HEK293 cells with 10 mM strontium as the charge carrier by the patch clamp technique. The kinetic properties and pharmacological features of ISr-HEK were characterized and compared with the properties of a heterologously expressed chimeric L-type calcium channel construct. 2. ISr-HEK activated on depolarization to voltages positive of -40 mV. It had transient current kinetics with a time to peak of 16 +/- 1.4 ms (n = 7) and an inactivation times constant of 52 +/- 5 ms (n = 7) at a test potential of 0 mV. The voltage for half maximal activation was -19.0 +/- 1.5 mV (n = 7) and the voltage for half maximal steady-state inactivation was -39.7 +/- 2.3 mV (n = 7). 3. Block of ISr-HEK by the dihydropyridine isradipine was not stereoselective; 1 microM (+) and (-)-isradipine inhibited the current by 30 +/- 4% (n = 3) and 29 +/- 2% (n = 4) respectively. (+)-Isradipine and (-)-isradipine (10 microM) inhibited ISr-HEK by 89 +/- 4% (n = 5) and 88 +/- 8% (n = 3) respectively. The 7-bromo substituted (+/-)-isradipine (VO2605, 10 microM) which is almost inactive on L-type calcium channels also inhibited ISr-HEK (83 +/- 9%, n = 3) as was observed for 10 microM (-)-nimodipine (78 +/- 6%, n = 5). Interestingly, 10 microM (+/-)-Bay K 8644 (n = 5) had no effect on the current. ISr-HEK was only slightly inhibited by the cone snail toxins omega-CTx GVIA (1 microM, inhibition by 17 +/- 3%, n = 4) and omega-CTx MVIIC (1 microM, inhibition by 20 +/- 3%, n = 4). The funnel web spider toxin omega-Aga IVA (200 nM) inhibited ISr-HEK by 19 +/- 2%, n = 4). 4. In cells expressing ISr-HEK, maximum inward current densities of 0.24 +/- 0.03 pA/pF and 0.39 +/- 0.7 pA/ pF (at a test potential of -10 mV) were estimated in two different batches of HEK293 cells. The current density increased to 0.88 +/- 0.18 pA/pF or 1.11 +/- 0.2 pA/pF respectively, if the cells were cultured for 4 days in serum-free medium. 5. Co-expression of a chimeric L-type calcium channel construct revealed that ISr-HEK and L-type calcium channel currents could be distinguished by their different voltage-dependencies and current kinetics. The current density after heterologous expression of the L-type alpha 1 subunit chimera was estimated to be about ten times higher in serum containing medium (2.14 +/- 0.45 pA/pF) than that of ISr-HEK under the same conditions.
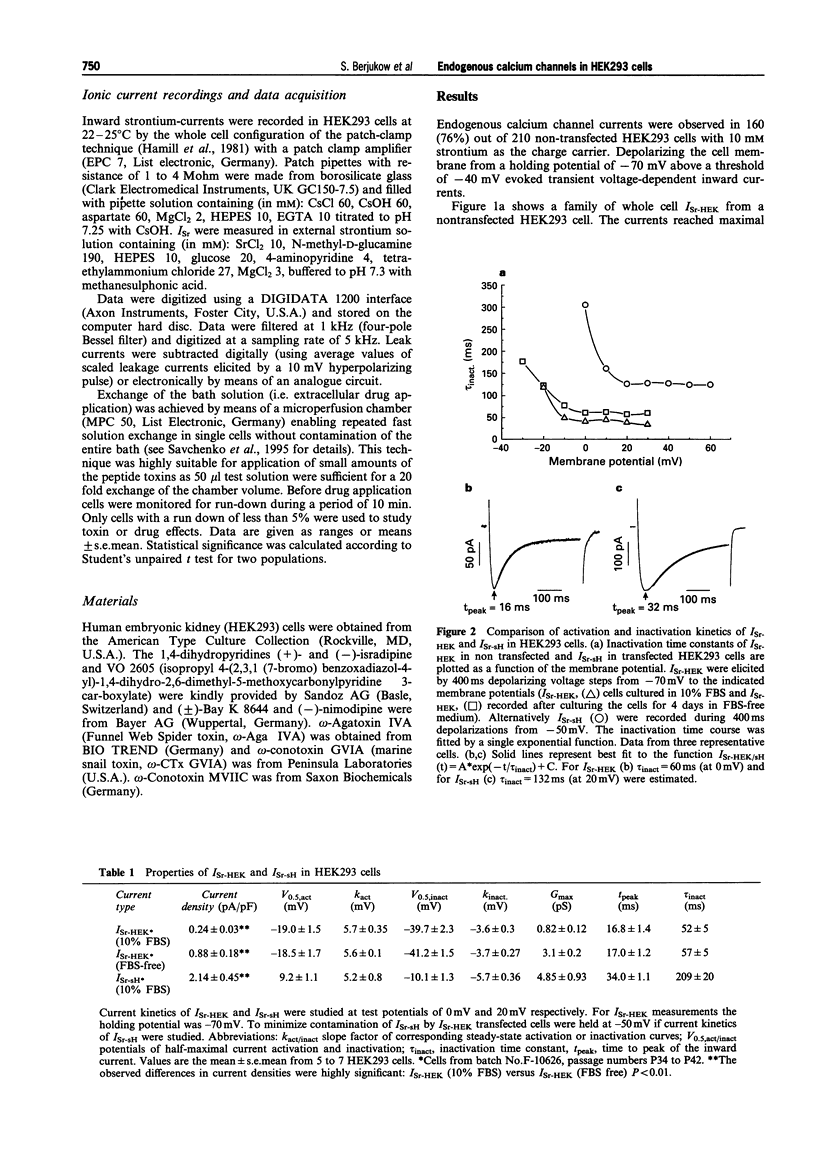
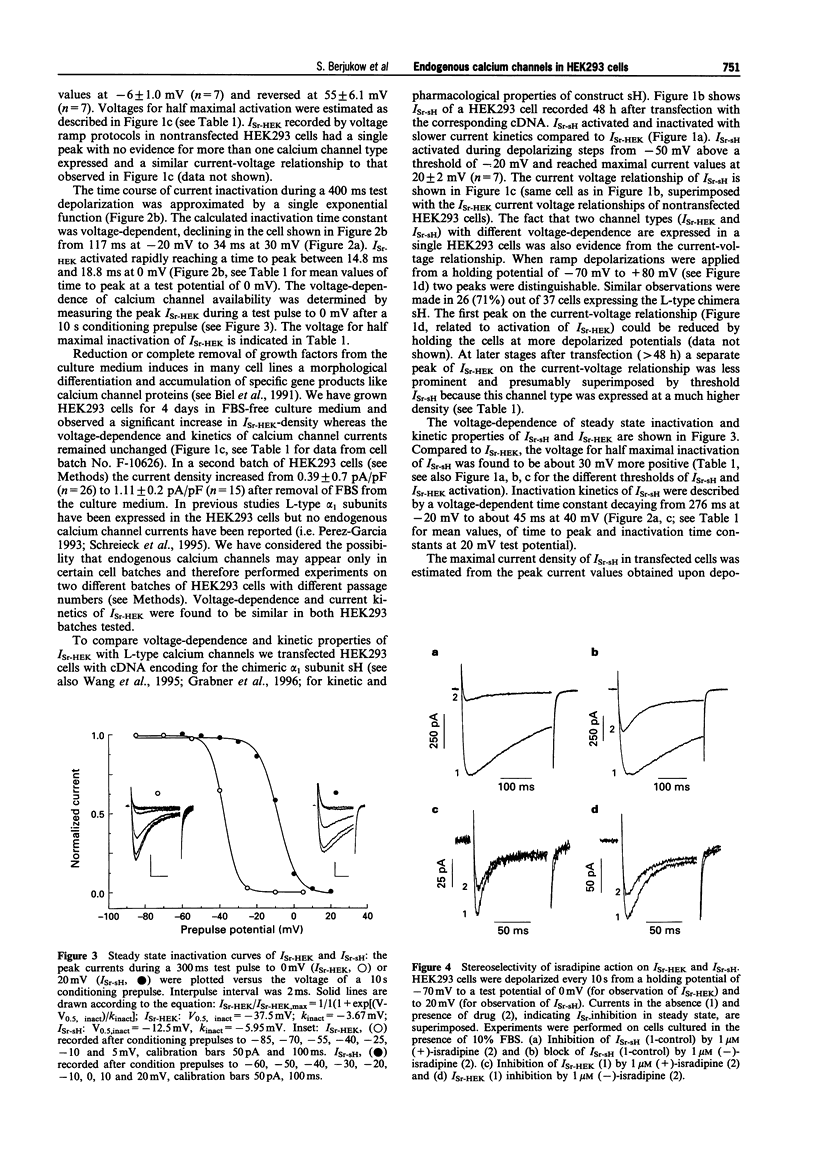
Full text
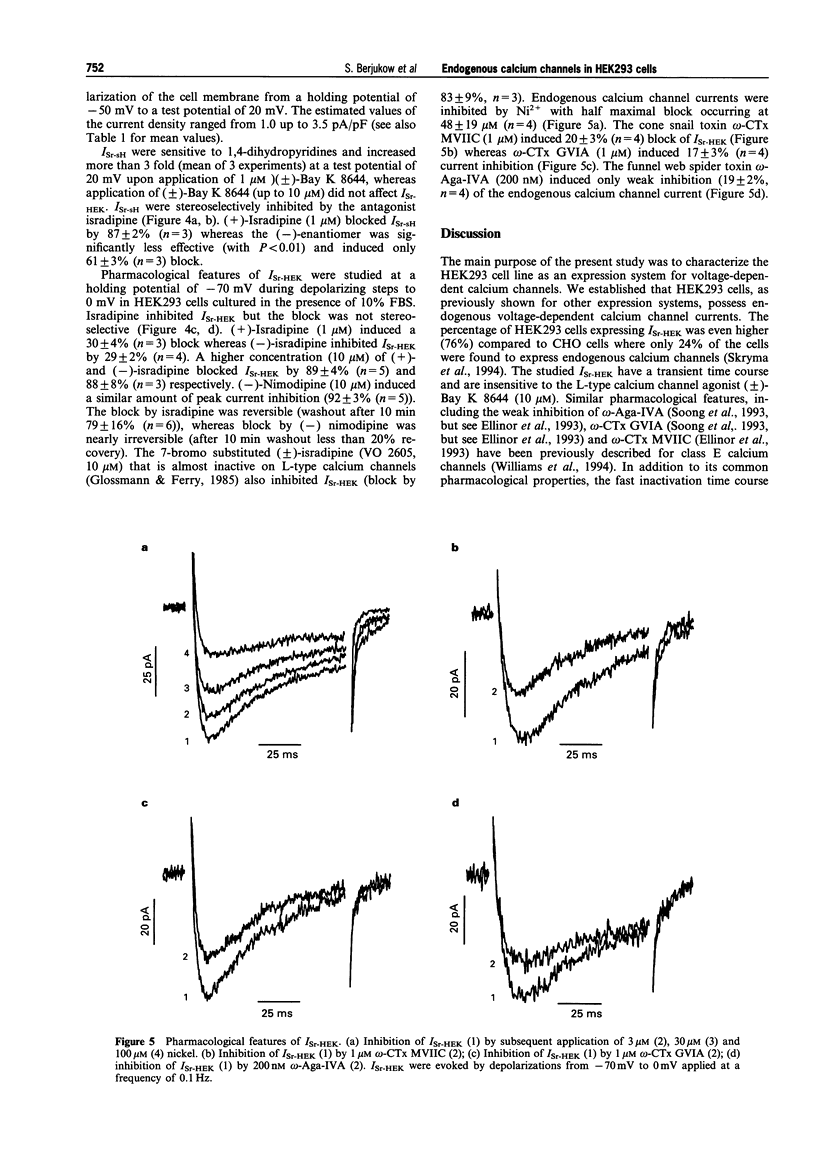
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. A novel calcium current in dysgenic skeletal muscle. J Gen Physiol. 1989 Sep;94(3):429–444. doi: 10.1085/jgp.94.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M., Hullin R., Freundner S., Singer D., Dascal N., Flockerzi V., Hofmann F. Tissue-specific expression of high-voltage-activated dihydropyridine-sensitive L-type calcium channels. Eur J Biochem. 1991 Aug 15;200(1):81–88. doi: 10.1111/j.1432-1033.1991.tb21051.x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bourinet E., Fournier F., Nargeot J., Charnet P. Endogenous Xenopus-oocyte Ca-channels are regulated by protein kinases A and C. FEBS Lett. 1992 Mar 24;299(1):5–9. doi: 10.1016/0014-5793(92)80087-w. [DOI] [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Grabner M., Friedrich K., Knaus H. G., Striessnig J., Scheffauer F., Staudinger R., Koch W. J., Schwartz A., Glossmann H. Calcium channels from Cyprinus carpio skeletal muscle. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):727–731. doi: 10.1073/pnas.88.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M., Wang Z., Hering S., Striessnig J., Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996 Jan;16(1):207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Perez-Reyes E., Wei X., Castellano A., Brown A. M. T-type and N-type calcium channels of Xenopus oocytes: evidence for specific interactions with beta subunits. Biophys J. 1994 Jun;66(6):1833–1843. doi: 10.1016/S0006-3495(94)80977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Kamp T. J., Marbán E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of alpha 1 and beta subunits. J Gen Physiol. 1995 Feb;105(2):289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Savchenko A., Glossmann H., Hering S. Improved micro-perfusion chamber for multiple and rapid solution exchange in adherent single cells. Pflugers Arch. 1995 Jan;429(3):436–442. doi: 10.1007/BF00374161. [DOI] [PubMed] [Google Scholar]
- Skryma R., Prevarskaya N., Vacher P., Dufy B. Voltage-dependent Ca2+ channels in Chinese hamster ovary (CHO) cells. FEBS Lett. 1994 Aug 1;349(2):289–294. doi: 10.1016/0014-5793(94)00690-3. [DOI] [PubMed] [Google Scholar]
- Soong T. W., Stea A., Hodson C. D., Dubel S. J., Vincent S. R., Snutch T. P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993 May 21;260(5111):1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Wang Z., Grabner M., Berjukow S., Savchenko A., Glossmann H., Hering S. Chimeric L-type Ca2+ channels expressed in Xenopus laevis oocytes reveal role of repeats III and IV in activation gating. J Physiol. 1995 Jul 1;486(Pt 1):131–137. doi: 10.1113/jphysiol.1995.sp020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. E., Marubio L. M., Deal C. R., Hans M., Brust P. F., Philipson L. H., Miller R. J., Johnson E. C., Harpold M. M., Ellis S. B. Structure and functional characterization of neuronal alpha 1E calcium channel subtypes. J Biol Chem. 1994 Sep 2;269(35):22347–22357. [PubMed] [Google Scholar]



