Abstract
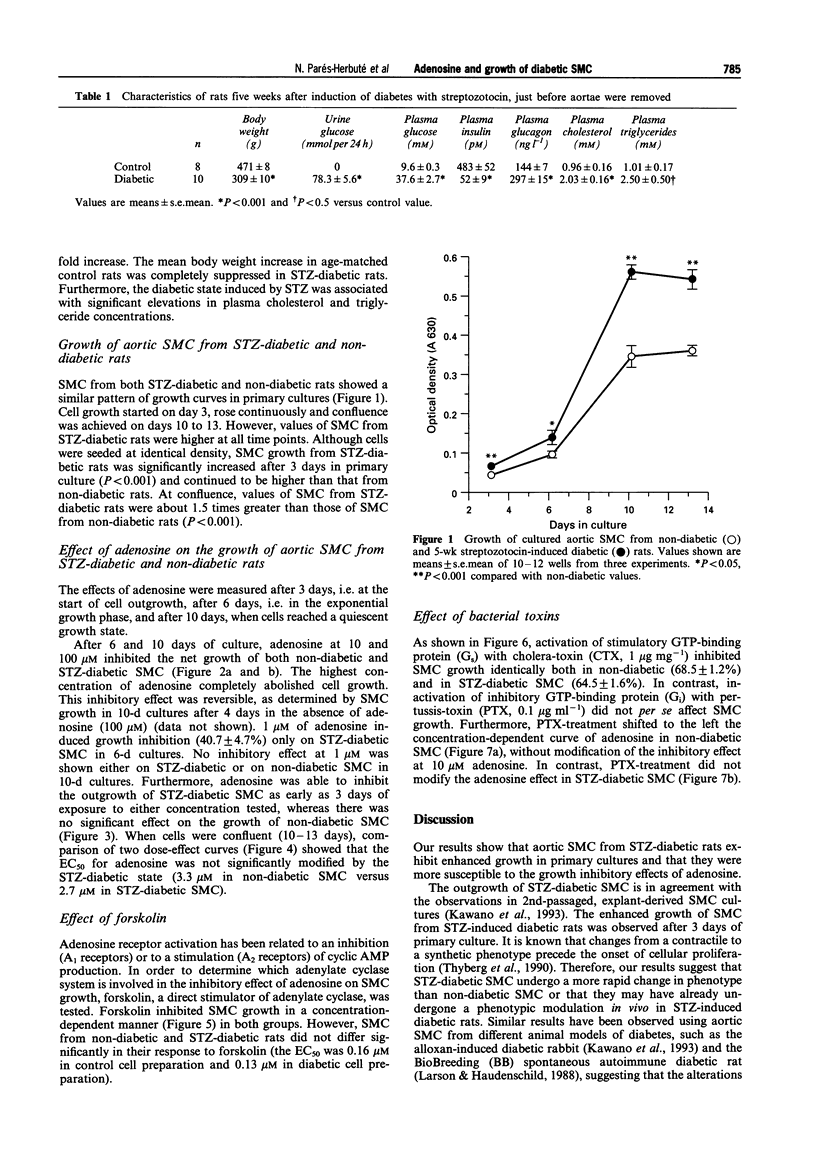
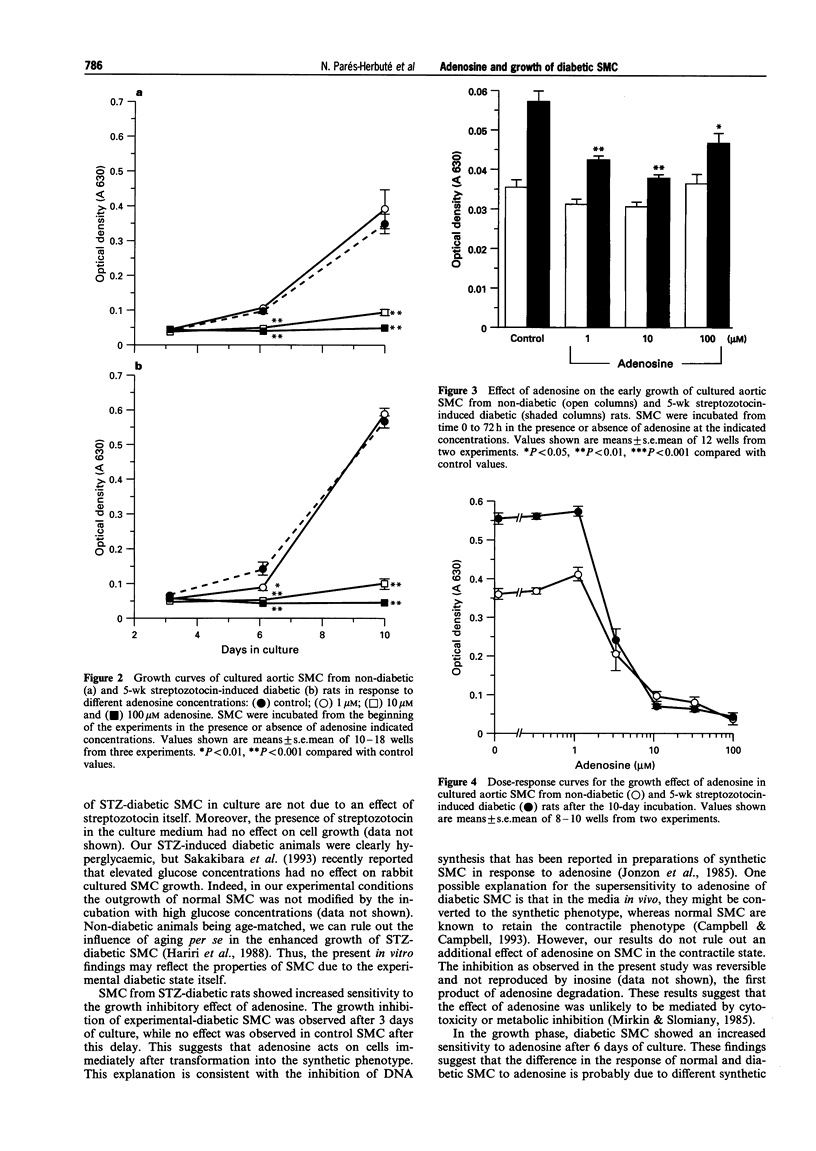
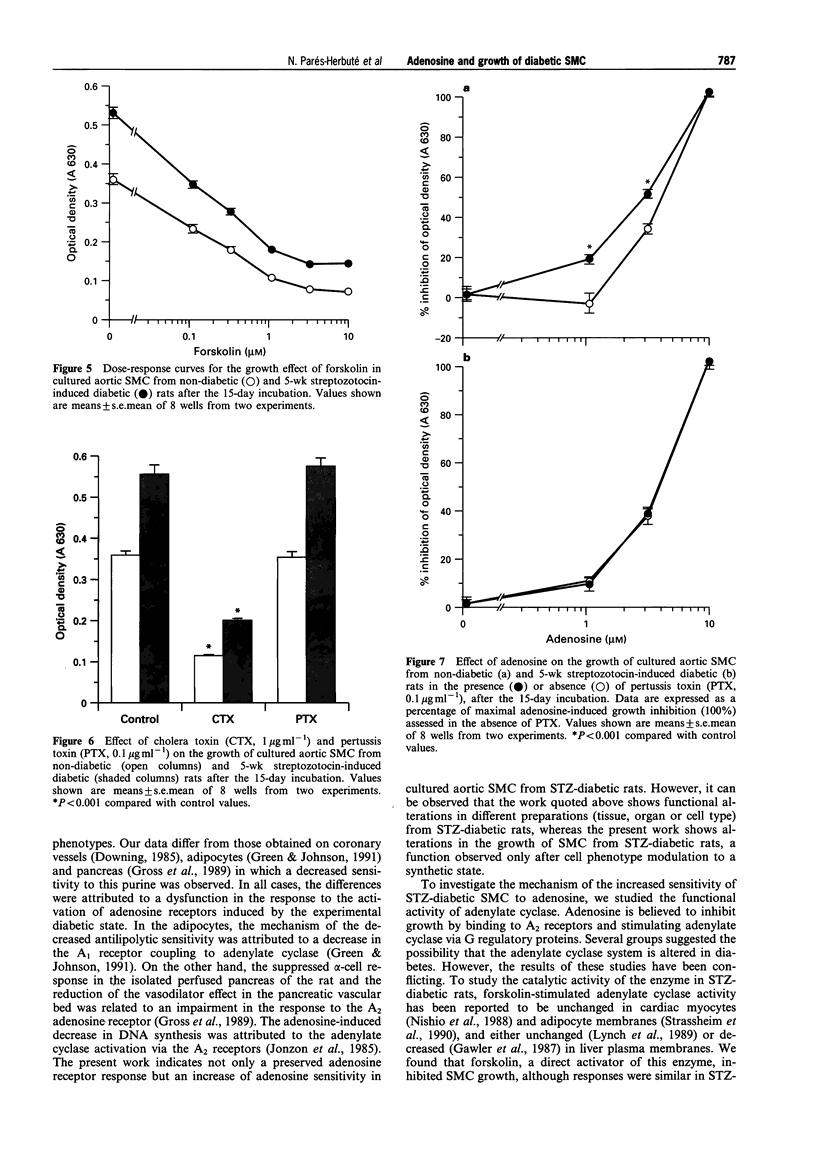
1. There is evidence to suggest that adenosine may regulate arterial smooth muscle cell (SMC) growth and proliferation, which is a key event in atherogenesis. This regulation may be mediated via adenylate cyclase. As diabetes is a known risk factor for atherosclerosis, we investigated the growth of aortic SMC from diabetic rats in primary culture and their sensitivity to adenosine and to adenylate cyclase activity. 2. Diabetes was induced with streptozotocin (STZ, 66 mg kg-1, i.p.) Aortic SMC primary cultures were prepared from STZ-diabetic and age-matched rats 5 weeks after the STZ injection. 3. SMC from STZ-diabetic rats grew faster and reached greater densities at confluence than those from non-diabetic animals. 4. Adenosine inhibited growth in both control and diabetic SMC. However, cells from STZ-diabetic rats were apparently more sensitive to adenosine. 5. Direct activation of adenylate cyclase by forskolin induced a dose-dependent growth inhibition, similar in both groups of cells. 6. Cholera toxin, an activator of stimulatory GTP-binding protein (Gs), induced a similar growth inhibitory response in non-diabetic and diabetic SMC. Pertussis toxin (PTX), an inactivator of inhibitory GTP-binding protein (Gi), did not itself affect SMC growth. However, PTX increased dose-dependently the growth inhibition induced by adenosine in SMC from non-diabetic rats but not in SMC from diabetic rats. 7. These findings suggest a functional abnormality in Gi activity in SMC from diabetic rats, that would explain the increased sensitivity to the nucleoside. This impaired inhibitory pathway may reflect changes in the growth regulation of SMC in experimental diabetic states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alric R., Mariani M. M., Loubatières A. Importance de l'état des éléments figurés du sang et en particulier de celui des globules rouges sur les valeurs du glucose sanguin mesurées par l'auto-analyseur Technicon. Pathol Biol. 1965 May;13(9):506–511. [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Campbell G. R. Culture techniques and their applications to studies of vascular smooth muscle. Clin Sci (Lond) 1993 Nov;85(5):501–513. doi: 10.1042/cs0850501. [DOI] [PubMed] [Google Scholar]
- Colwell J. A., Lopes-Virella M. F. A review of the development of large-vessel disease in diabetes mellitus. Am J Med. 1988 Nov 28;85(5A):113–118. doi: 10.1016/0002-9343(88)90403-2. [DOI] [PubMed] [Google Scholar]
- Cros G. H., Chanez P. O., Michel A., McNeill J. H., Serrano J. J. Cardiac beta-adrenergic receptors in diabetic rats: alteration of guanyl nucleotide regulation. J Pharmacol. 1986 Oct-Dec;17(4):595–600. [PubMed] [Google Scholar]
- Downing S. E. Restoration of coronary dilator action of adenosine in experimental diabetes. Am J Physiol. 1985 Jul;249(1 Pt 2):H102–H107. doi: 10.1152/ajpheart.1985.249.1.H102. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Green A., Johnson J. L. Evidence for impaired coupling of receptors to Gi protein in adipocytes from streptozocin-induced diabetic rats. Diabetes. 1991 Jan;40(1):88–94. doi: 10.2337/diab.40.1.88. [DOI] [PubMed] [Google Scholar]
- Gross R., Hillaire-Buys D., Bertrand G., Ribes G., Loubatieres-Mariani M. M. Diabetes and impaired response of glucagon cells and vascular bed to adenosine in rat pancreas. Diabetes. 1989 Oct;38(10):1291–1295. doi: 10.2337/diab.38.10.1291. [DOI] [PubMed] [Google Scholar]
- Hariri R. J., Hajjar D. P., Coletti D., Alonso D. R., Weksler M. E., Rabellino E. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988 Apr;131(1):132–136. [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Hanski E., Houslay M. D. Islet-activating protein blocks glucagon desensitization in intact hepatocytes. Biochem J. 1984 Aug 15;222(1):189–194. doi: 10.1042/bj2220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. B., Dall'Aglio E., Hollenbeck C., Chang H., Reaven G. M. Suppression of free fatty acids and triglycerides in normal and hypertriglyceridemic rats by the adenosine receptor agonist phenylisopropyladenosine. J Pharmacol Exp Ther. 1986 Dec;239(3):715–718. [PubMed] [Google Scholar]
- Jonzon B., Nilsson J., Fredholm B. B. Adenosine receptor-mediated changes in cyclic AMP production and DNA synthesis in cultured arterial smooth muscle cells. J Cell Physiol. 1985 Sep;124(3):451–456. doi: 10.1002/jcp.1041240314. [DOI] [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Kawano M., Koshikawa T., Kanzaki T., Morisaki N., Saito Y., Yoshida S. Diabetes mellitus induces accelerated growth of aortic smooth muscle cells: association with overexpression of PDGF beta-receptors. Eur J Clin Invest. 1993 Feb;23(2):84–90. doi: 10.1111/j.1365-2362.1993.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Haudenschild C. C. Activation of smooth muscle cell outgrowth from BB/Wor rat aortas. Diabetes. 1988 Oct;37(10):1380–1385. doi: 10.2337/diab.37.10.1380. [DOI] [PubMed] [Google Scholar]
- Linden J., Tucker A. L., Lynch K. R. Molecular cloning of adenosine A1 and A2 receptors. Trends Pharmacol Sci. 1991 Sep;12(9):326–328. doi: 10.1016/0165-6147(91)90589-k. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Blackmore P. F., Johnson E. H., Wange R. L., Krone P. K., Exton J. H. Guanine nucleotide binding regulatory proteins and adenylate cyclase in livers of streptozotocin- and BB/Wor-diabetic rats. Immunodetection of Gs and Gi with antisera prepared against synthetic peptides. J Clin Invest. 1989 Jun;83(6):2050–2062. doi: 10.1172/JCI114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias A., Albino-Teixeira A., Polónia J., Azevedo I. Long-term administration of 1,3-dipropyl-8-sulfophenylxanthine causes arterial hypertension. Eur J Pharmacol. 1991 Jan 25;193(1):101–104. doi: 10.1016/0014-2999(91)90206-6. [DOI] [PubMed] [Google Scholar]
- Morrison P. D., Mackinnon M. W., Bartrup J. T., Skett P. G., Stone T. W. Changes in adenosine sensitivity in the hippocampus of rats with streptozotocin-induced diabetes. Br J Pharmacol. 1992 Apr;105(4):1004–1008. doi: 10.1111/j.1476-5381.1992.tb09092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Southgate K. M., Assender J. W. Inhibition of vascular smooth muscle cell proliferation by endothelium-dependent vasodilators. Herz. 1992 Oct;17(5):291–299. [PubMed] [Google Scholar]
- Nishio Y., Kashiwagi A., Kida Y., Kodama M., Abe N., Saeki Y., Shigeta Y. Deficiency of cardiac beta-adrenergic receptor in streptozocin-induced diabetic rats. Diabetes. 1988 Sep;37(9):1181–1187. doi: 10.2337/diab.37.9.1181. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Haudenschild C. Diabetes as an atherogenic factor. Prog Cardiovasc Dis. 1984 Mar-Apr;26(5):373–412. doi: 10.1016/0033-0620(84)90011-2. [DOI] [PubMed] [Google Scholar]
- Rutkiewicz J., Górski J. On the role of insulin in regulation of adenosine deaminase activity in rat tissues. FEBS Lett. 1990 Oct 1;271(1-2):79–80. doi: 10.1016/0014-5793(90)80376-t. [DOI] [PubMed] [Google Scholar]
- Sakakibara F., Hotta N., Koh N., Sakamoto N. Effects of high glucose concentrations and epalrestat on sorbitol and myo-inositol metabolism in cultured rabbit aortic smooth muscle cells. Diabetes. 1993 Nov;42(11):1594–1600. doi: 10.2337/diab.42.11.1594. [DOI] [PubMed] [Google Scholar]
- Strassheim D., Milligan G., Houslay M. D. Diabetes abolishes the GTP-dependent, but not the receptor-dependent inhibitory function of the inhibitory guanine-nucleotide-binding regulatory protein (Gi) on adipocyte adenylate cyclase activity. Biochem J. 1990 Mar 1;266(2):521–526. doi: 10.1042/bj2660521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjölund M., Palmberg L., Bottger B. A. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990 Nov-Dec;10(6):966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


