Abstract
1. The effects of levcromakalim (BRL 38227) on ionic currents recorded from pig proximal urethra were investigated by use of tension measurement and patch clamp techniques (conventional whole-cell configuration, nystatin perforated patch, and cell-attached configuration). 2. Levcromakalim (1 microM) caused a relaxation in the resting tone. This levcromakalim-induced relaxation was inhibited by the pretreatment with 1 microM glibenclamide. 3. The resting membrane potential recorded from single cells in current-clamp mode was-36.1 +/- 4.4 mV (n = 5). 4. Levcromakalim induced a concentration-dependent hyperpolarization with a maximum (at > or = 10 microM) close to the theoretical equilibrium potential of potassium (EK). The membrane hyperpolarization caused by 1 microM levcromakalim (24.7 +/- 5.8 mV, n = 4) was abolished by 1 microM glibenclamide. 5. Levcromakalim (100 microM) caused an outward K current in whole-cell recordings which was unaffected by iberiotoxin (300 nM) but abolished by glibenclamide (10 microM). 6. In cell-attached patches, levcromakalim activated a 43 pS K channel which was inhibited by the application of glibenclamide. 7. The metabolic poison, cyanide (CN), also activated a 43 pS K channel which was suppressed by the application of 10 microM glibenclamide. 8. These results indicate that levcromakalim and metabolic inhibition activate the same 43 pS K channel in pig proximal urethra. The resultant urethral hyperpolarization might reduce the usefulness of K channel openers in the treatment of detrusor instability, but be of value in treating outflow obstruction.
Full text
PDF

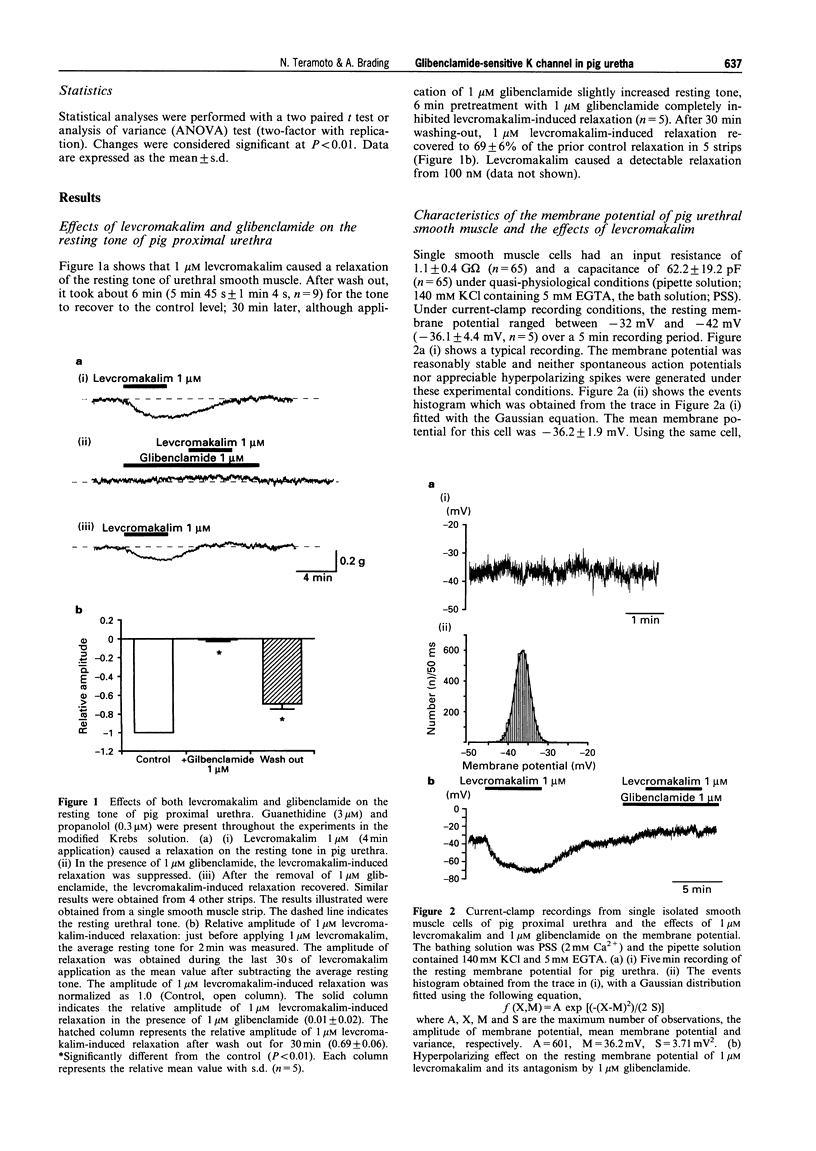
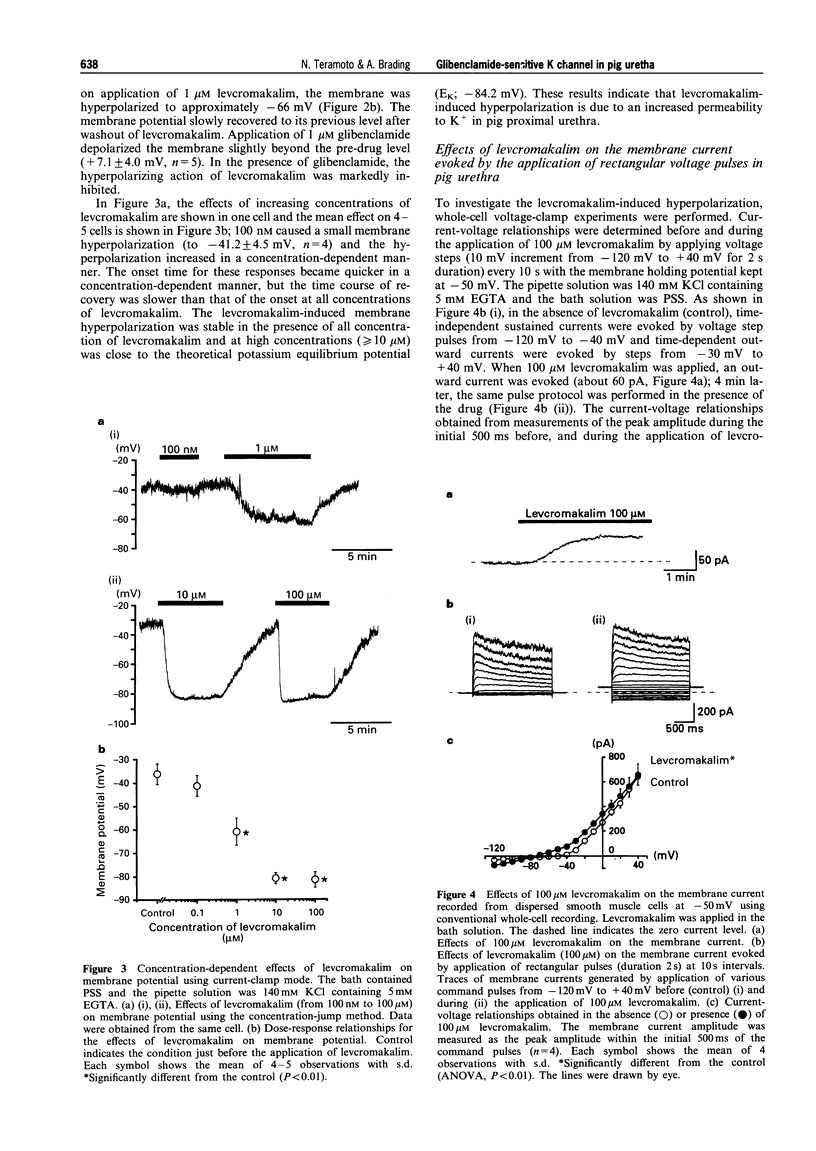


Selected References
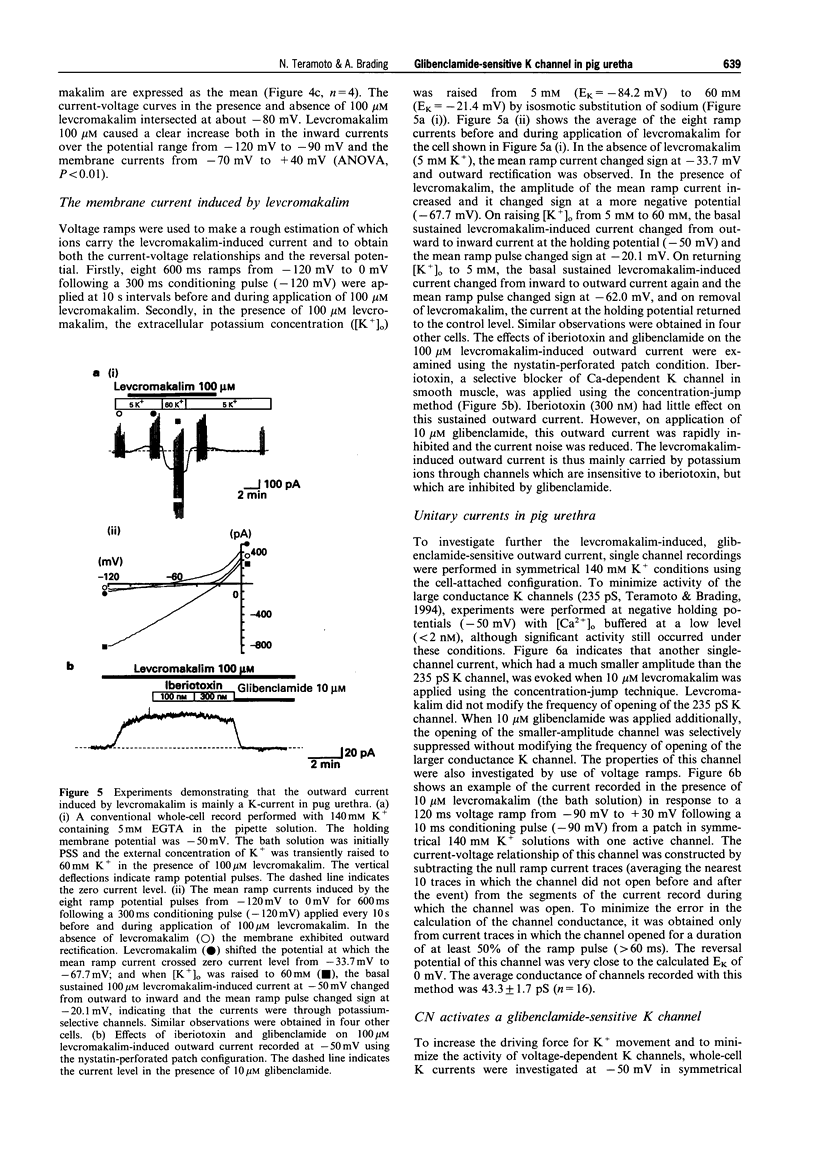
These references are in PubMed. This may not be the complete list of references from this article.
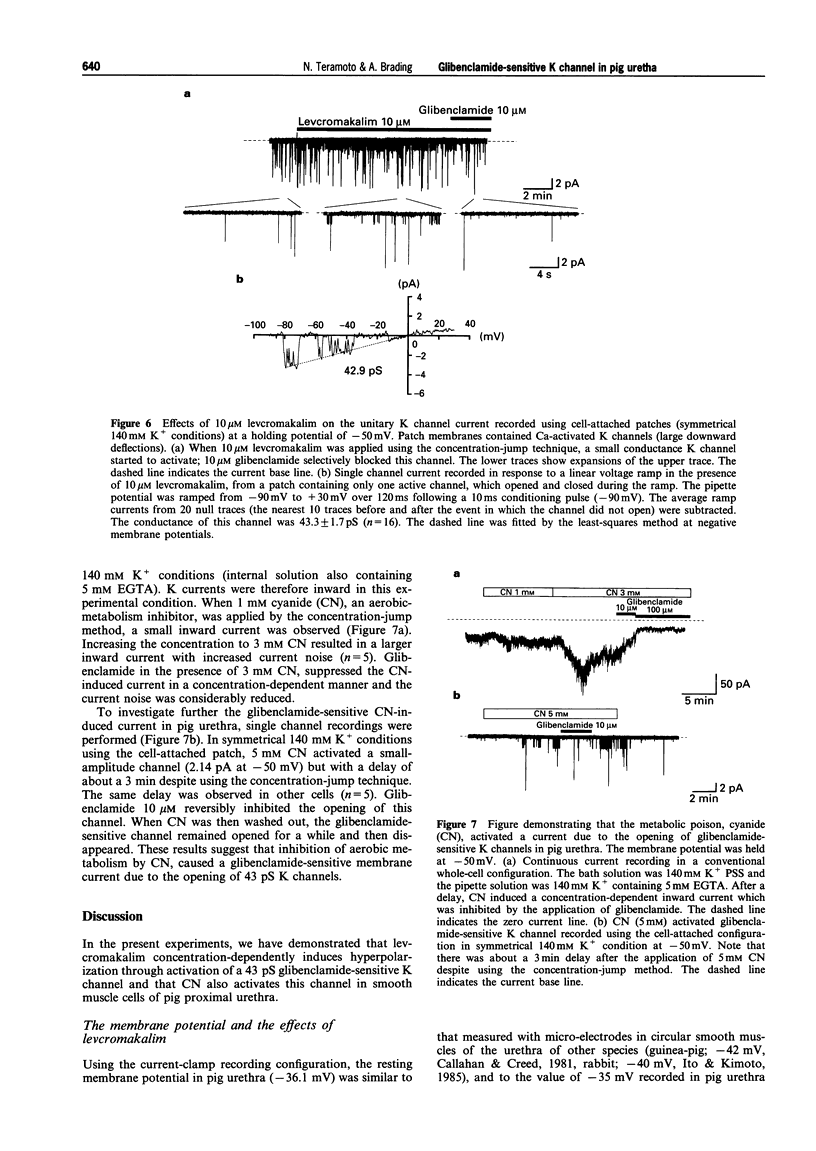
- Anderson K. E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993 Sep;45(3):253–308. [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993 Oct;110(2):573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Turner W. H. The unstable bladder: towards a common mechanism. Br J Urol. 1994 Jan;73(1):3–8. doi: 10.1111/j.1464-410x.1994.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Callahan S. M., Creed K. E. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol. 1981 Oct;74(2):353–358. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dart C., Standen N. B. Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1995 Feb 15;483(Pt 1):29–39. doi: 10.1113/jphysiol.1995.sp020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Foster C. D., Fujii K., Kingdon J., Brading A. F. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol. 1989 May;97(1):281–291. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. D., Speakman M. J., Fujii K., Brading A. F. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989 Mar;63(3):284–294. doi: 10.1111/j.1464-410x.1989.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Fujii K., Foster C. D., Brading A. F., Parekh A. B. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990 Apr;99(4):779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedlund H., Mattiasson A., Andersson K. E. Effects of pinacidil on detrusor instability in men with bladder outlet obstruction. J Urol. 1991 Nov;146(5):1345–1347. doi: 10.1016/s0022-5347(17)38087-4. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kimoto Y. The neural and non-neural mechanisms involved in urethral activity in rabbits. J Physiol. 1985 Oct;367:57–72. doi: 10.1113/jphysiol.1985.sp015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani H., Ginkawa M., Sakai T. A simple method for measurement of ureteric peristaltic function in vivo and the effects of drugs acting on ion channels applied from the ureter lumen in anesthetized rats. Jpn J Pharmacol. 1993 Aug;62(4):331–338. doi: 10.1254/jjp.62.331. [DOI] [PubMed] [Google Scholar]
- Kontani H., Jinkawa M., Sakai T. Effects of levcromakalim on ureteral peristaltic function and cystometrogram in rats. Jpn J Pharmacol. 1993 Dec;63(4):503–511. doi: 10.1254/jjp.63.503. [DOI] [PubMed] [Google Scholar]
- MELICK W. F., NARYKA J. J., SCHMIDT J. H. Experimental studies of ureteral peristaltic patterns in the pig. I. Similarity of pig and human ureter and bladder physiology. J Urol. 1961 Feb;85:145–148. doi: 10.1016/S0022-5347(17)65296-0. [DOI] [PubMed] [Google Scholar]
- Malmgren A., Andersson K. E., Sjögren C., Andersson P. O. Effects of pinacidil and cromakalim (BRL 34915) on bladder function in rats with detrusor instability. J Urol. 1989 Oct;142(4):1134–1138. doi: 10.1016/s0022-5347(17)39012-2. [DOI] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K., Okabe K., Kitamura K., Kuriyama H., Weston A. H. Characteristics of cromakalim-induced relaxations in the smooth muscle cells of guinea-pig mesenteric artery and vein. Br J Pharmacol. 1988 Nov;95(3):795–804. doi: 10.1111/j.1476-5381.1988.tb11707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Paul R. J. Smooth muscle energetics. Annu Rev Physiol. 1989;51:331–349. doi: 10.1146/annurev.ph.51.030189.001555. [DOI] [PubMed] [Google Scholar]
- Russell S. N., Smirnov S. V., Aaronson P. I. Effects of BRL 38227 on potassium currents in smooth muscle cells isolated from rabbit portal vein and human mesenteric artery. Br J Pharmacol. 1992 Mar;105(3):549–556. doi: 10.1111/j.1476-5381.1992.tb09017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki N., Karim O. M., Mostwin J. L. Effect of pinacidil on the membrane electrical activity of guinea pig detrusor muscle. J Pharmacol Exp Ther. 1992 Nov;263(2):816–822. [PubMed] [Google Scholar]
- Silberberg S. D., van Breemen C. A potassium current activated by lemakalim and metabolic inhibition in rabbit mesenteric artery. Pflugers Arch. 1992 Jan;420(1):118–120. doi: 10.1007/BF00378653. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Bolton T. B. Activation by intracellular GDP, metabolic inhibition and pinacidil of a glibenclamide-sensitive K-channel in smooth muscle cells of rat mesenteric artery. Br J Pharmacol. 1995 Feb;114(3):662–672. doi: 10.1111/j.1476-5381.1995.tb17190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


