Abstract
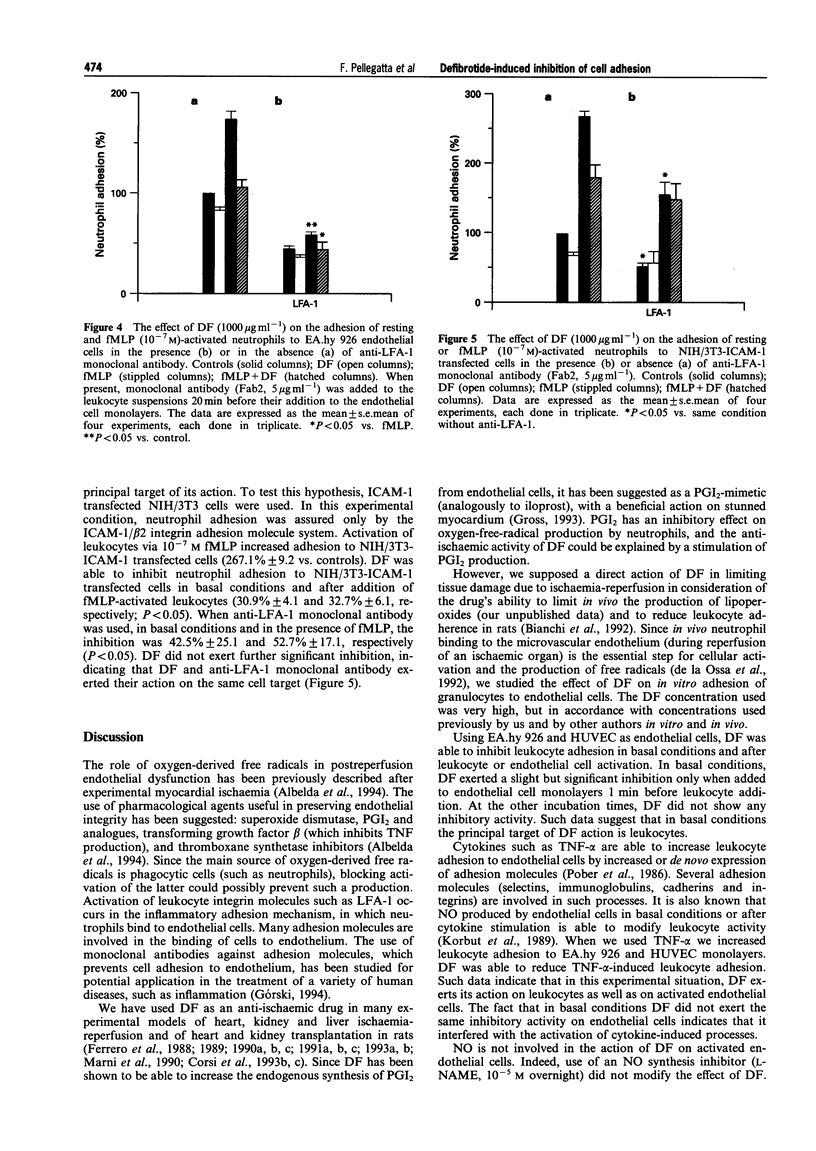
1. Leukocyte-endothelial cell interactions play an important role during ischaemia-reperfusion events. Adhesion molecules are specifically implicated in this interaction process. 2. Since defibrotide has been shown to be an efficient drug in reducing damage due to ischaemia-reperfusion in many experimental models, we analysed the effect of defibrotide in vitro on leukocyte adhesion to endothelial cells in basal conditions and after their stimulation. 3. In basal conditions, defibrotide (1000 micrograms ml-1) partially inhibited leukocyte adhesion to endothelial cells by 17.3% +/- 3.6 (P < 0.05), and after endothelial cell stimulation (TNF-alpha, 500 u ml-1) or after leukocyte stimulation (fMLP, 10(-7) M), it inhibited leukocyte adhesion by 26.5% +/- 3.4 and 32.4% +/- 1.8, respectively (P < 0.05). 4. In adhesion blockage experiments, the use of the monoclonal antibody anti-CD31 (5 micrograms ml-1) did not demonstrate a significant inhibitory effect whereas use of the monoclonal antibody anti-LFA-1 (5 micrograms ml-1) significantly interfered with the effect of defibrotide. 5. This result was confirmed in NIH/3T3-ICAM-1 transfected cells. 6. We conclude that defibrotide is able to interfere with leukocyte adhesion to endothelial cells mainly in activated conditions and that the ICAM-1/LFA-1 adhesion system is involved in the defibrotide mechanism of action.
Full text
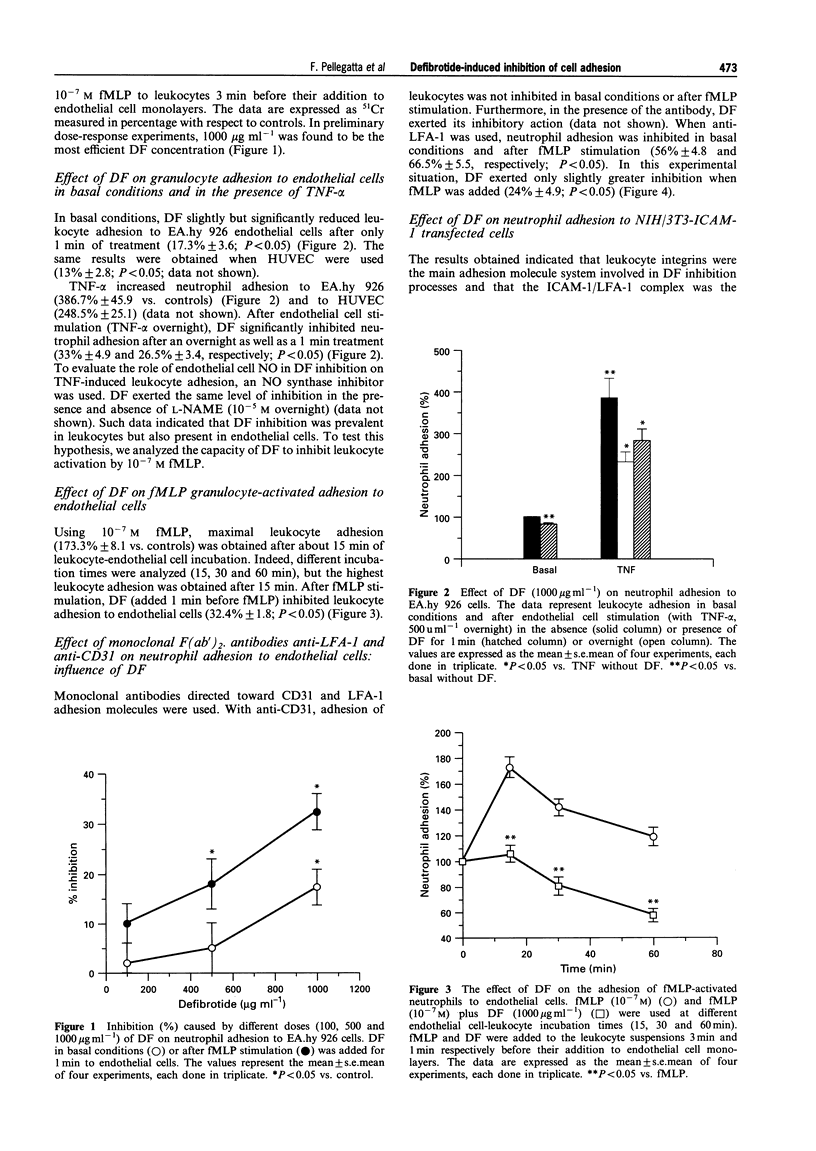
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Smith C. W., Ward P. A. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–512. [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Corsi M., Marni A., Gaja G., Ferrero M. E. Prolonged survival of grafted rat hearts by use of defibrotide and cyclosporine. Transplant Proc. 1993 Dec;25(6):3281–3282. [PubMed] [Google Scholar]
- Corsi M., Parise M., Gaja G., Ferrero M. E. Antiischaemic effect of defibrotide treatment in rat kidney. Drugs Exp Clin Res. 1993;19(6):261–265. [PubMed] [Google Scholar]
- Corsi M., Parise M., Gaja G., Ferrero M. E. Possible role of defibrotide in endothelial cell protection. Int J Tissue React. 1993;15(4):157–161. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Ferrero M. E., Corsi M., Parise M., Marni A., Gaja G. Defibrotide use during preservation favors the metabolic function of grafted kidneys in rats. Transplant Proc. 1993 Aug;25(4):2531–2533. [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Aimini R., Bergamasco E., Gaja G. Improved metabolic function of rat heart during procurement using defibrotide. Transplant Proc. 1990 Oct;22(5):2230–2232. [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Corsi M., Gaja G. Improved grafted rat heart metabolic function using defibrotide. Transplant Proc. 1993 Dec;25(6):3222–3224. [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Gaja G. Defibrotide, a profibrinolytic drug, reduces damage due to post-ischaemic reperfusion in rats. Biochem Soc Trans. 1990 Jun;18(3):501–502. doi: 10.1042/bst0180501. [DOI] [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Gaja G. Prevention of impaired liver metabolism due to ischemia in rats. Efficacy of defibrotide administration. J Hepatol. 1990 Mar;10(2):223–227. doi: 10.1016/0168-8278(90)90056-w. [DOI] [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Parise M., Salari P. C., Gaja G. Protection of rat heart from damage due to ischemia-reperfusion during procurement and grafting by defibrotide. Transplantation. 1991 Oct;52(4):611–615. doi: 10.1097/00007890-199110000-00006. [DOI] [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Rovati M., Aimini R., Salari P. C., Gaja G. Defibrotide, a stimulator of prostacyclin (PGI2) production, prevents the effects of ischaemic damage. Int J Tissue React. 1989;11(4):179–184. [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Salari P. C., Parise M., Spaggiari P. G., Gaja G. Usefulness of defibrotide treatment during organ procurement and preservation in rats. Transplant Proc. 1991 Oct;23(5):2359–2361. [PubMed] [Google Scholar]
- Ferrero M. E., Marni A., Salari P. C., Spaggiari P. G., Gaja G. Prostacyclin release from endothelial cells, induced by defibrotide treatment, favours the function of grafted rat hearts and kidneys. Int J Tissue React. 1991;13(4):215–218. [PubMed] [Google Scholar]
- Fischer A., Griscelli C., Blanche S., Le Deist F., Veber F., Lopez M., Delaage M., Olive D., Mawas C., Janossy G. Prevention of graft failure by an anti-HLFA-1 monoclonal antibody in HLA-mismatched bone-marrow transplantation. Lancet. 1986 Nov 8;2(8515):1058–1061. doi: 10.1016/s0140-6736(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Marcinkiewicz E., Radomski M., Swies J. Prostacyclin and the mechanism of action of defibrotide. Eicosanoids. 1989;2(3):163–167. [PubMed] [Google Scholar]
- Górski A. The role of cell adhesion molecules in immunopathology. Immunol Today. 1994 Jun;15(6):251–255. doi: 10.1016/0167-5699(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Korbut R., Trabka-Janik E., Gryglewski R. J. Cytoprotection of human polymorphonuclear leukocytes by stimulators of adenylate and guanylate cyclases. Eur J Pharmacol. 1989 Jun 8;165(1):171–172. doi: 10.1016/0014-2999(89)90786-3. [DOI] [PubMed] [Google Scholar]
- Kozar R. A., McKeone B. J., Pownall H. J. Free radical-induced alterations in endothelial cell function. J Surg Res. 1994 Jan;56(1):32–36. doi: 10.1006/jsre.1994.1006. [DOI] [PubMed] [Google Scholar]
- Kukielka G. L., Hawkins H. K., Michael L., Manning A. M., Youker K., Lane C., Entman M. L., Smith C. W., Anderson D. C. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993 Sep;92(3):1504–1516. doi: 10.1172/JCI116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mauff B., Hourmant M., Rougier J. P., Hirn M., Dantal J., Baatard R., Cantarovich D., Jacques Y., Soulillou J. P. Effect of anti-LFA1 (CD11a) monoclonal antibodies in acute rejection in human kidney transplantation. Transplantation. 1991 Aug;52(2):291–296. doi: 10.1097/00007890-199108000-00020. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Imhof B. A. Cell adhesion in the immune system. Immunol Today. 1993 Mar;14(3):99–102. doi: 10.1016/0167-5699(93)90205-Y. [DOI] [PubMed] [Google Scholar]
- Marni A., Ferrero M. E., Rovati M., Salari P. C., Gaja G. Protection of kidney from postischemic reperfusion injury in rats treated with defibrotide. Transplant Proc. 1990 Oct;22(5):2226–2229. [PubMed] [Google Scholar]
- Niada R., Pescador R., Porta R., Mantovani M., Prino G. Defibrotide is antithrombotic and thrombolytic against rabbit venous thrombosis. Haemostasis. 1986;16 (Suppl 1):3–8. doi: 10.1159/000215331. [DOI] [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Pescador R., Mantovani M., Prino G., Madonna M. Pharmacokinetics of Defibrotide and of its profibrinolytic activity in the rabbit. Thromb Res. 1983 Apr 1;30(1):1–11. doi: 10.1016/0049-3848(83)90391-2. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporciyan A. A., DeLisser H. M., Yan H. C., Mendiguren I. I., Thom S. R., Jones M. L., Ward P. A., Albelda S. M. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993 Dec 3;262(5139):1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- de la Ossa J. C., Malago M., Gewertz B. L. Neutrophil-endothelial cell binding in neutrophil-mediated tissue injury. J Surg Res. 1992 Jul;53(1):103–107. doi: 10.1016/0022-4804(92)90020-z. [DOI] [PubMed] [Google Scholar]


