Abstract
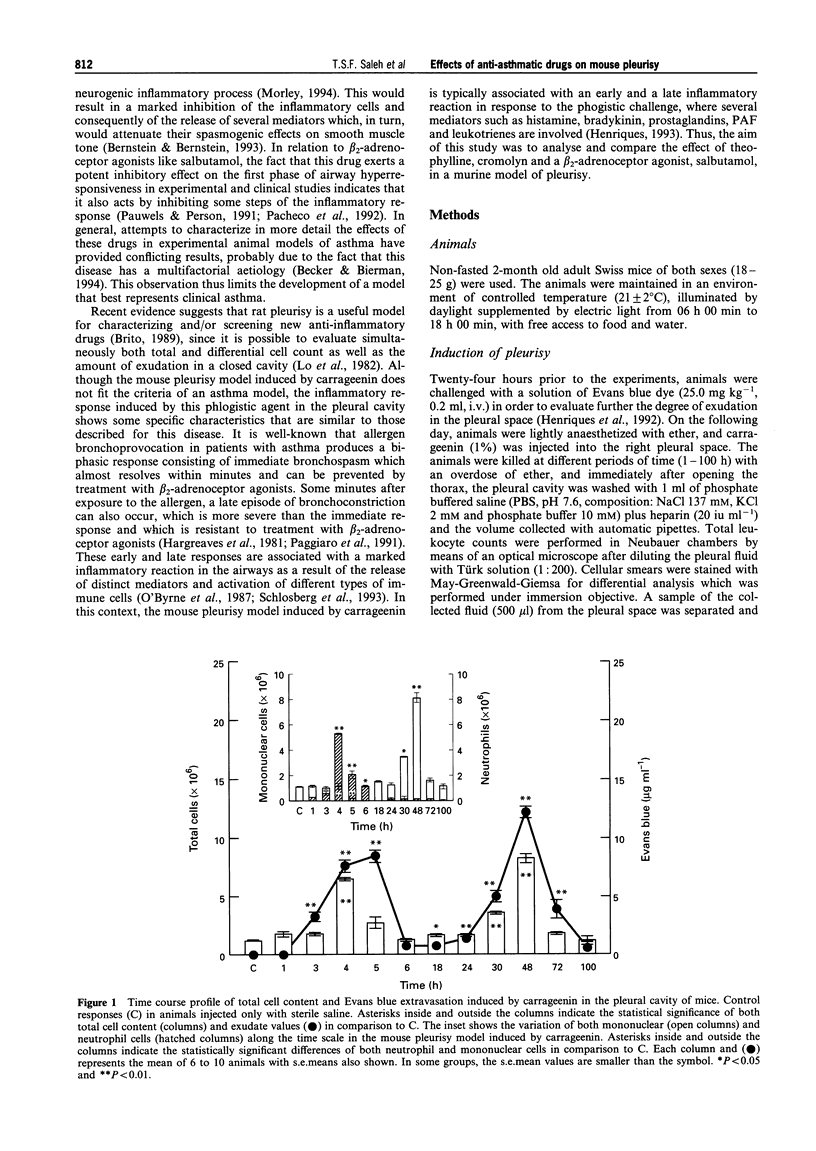
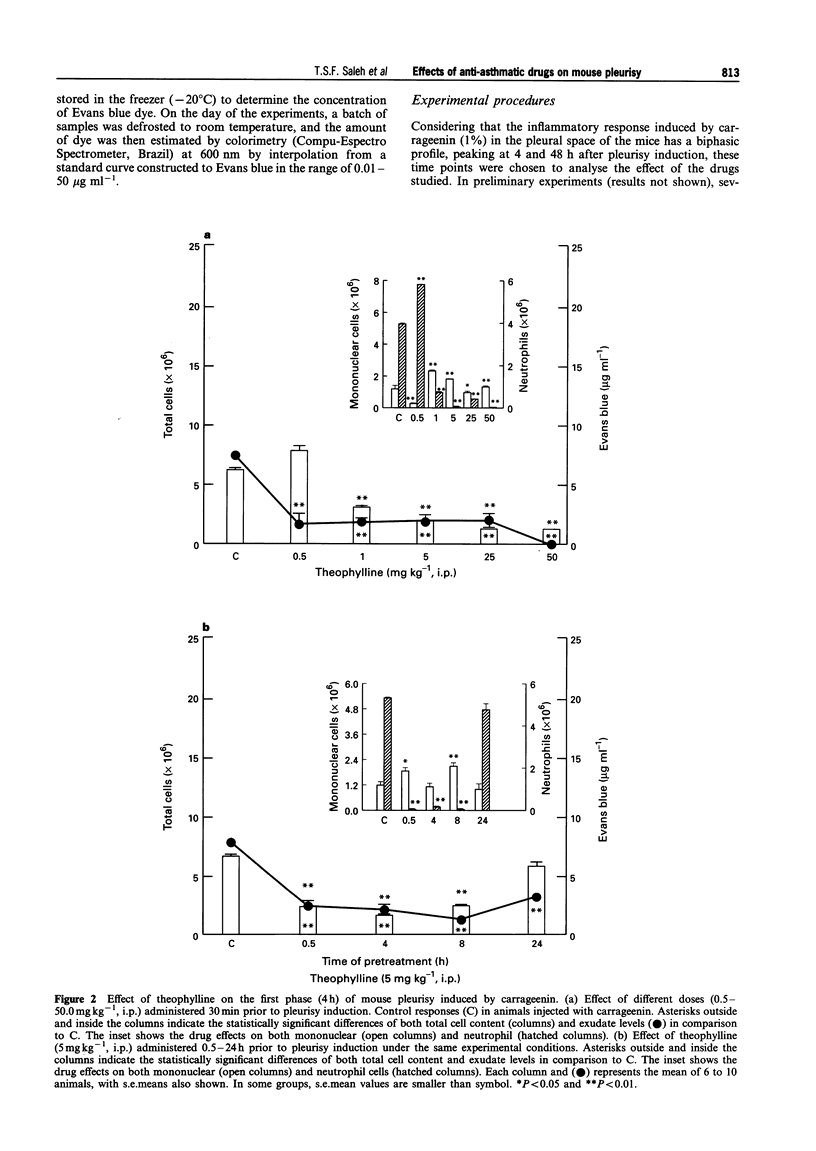
1. The aim of this study was to examine the effect of theophylline, cromolyn and salbutamol, three well-known anti-asthmatic drugs, on the early (4 h) and late (48 h) phases of cell migration and fluid leakage induced by carrageenin in the pleural cavity of mice. 2. In the first set of experiments, animals were pretreated (30 min) with different doses of theophylline (0.5-50 mg kg-1, i.p.), cromolyn (0.02-0.2 mg per pleural cavity) or salbutamol (0.05-50 mg kg-1, i.p.); the total and differential cell content, and also the exudate were analysed 4 h after carrageenin (1%) administration. Afterwards, in order to evaluate the time course effects of these drugs on both phases of the inflammatory reaction, one dose employed in the above protocol was chosen, to pretreat (0.5-24 h) different groups of animals. The studied parameters were evaluated 4 and 48 h after pleurisy induction. 3. Acute administration of theophylline (1-50 mg kg-1, i.p.) cromolyn (0.02-0.2 mg per pleural cavity) and salbutamol (0.5-50 mg kg-1, i.p.), 30 min prior to carrageenin, caused significant inhibition of total cell and fluid leakage in the pleural cavity at 4 h (P < 0.01). All drugs exerted a long-lasting inhibitory effect on both exudation and cell migration (P < 0.01) when administered 0.5-8 h before pleurisy induction. However, the temporal profile of the inhibitory effect induced by these drugs on the first phase of the inflammatory reaction was clearly different. Thus, the inhibitory effect induced by theophylline and cromolyn on exudation was significantly longer (up to 24 h) in comparison to their effects on cell migration (only up to 8 h). In contrast, although salbutamol when administered 30 min before pleurisy induction abolished fluid leakage (P < 0.01), this effect was not sustained in the groups pretreated for 4-8 h. In these latter groups, a significant but much smaller reduction of exudation was observed (P < 0.01), whereas the magnitude of cell migration inhibition did not vary. 4. The second phase (48 h) of the inflammatory reaction induced by carrageenin (1%) was significantly inhibited by cromolyn (0.02 mg per pleural cavity) when this drug was administered 0.5-24 h before pleurisy induction (P < 0.01). Similar results were observed when theophylline (50 mg kg-1, i.p.) was administered 0.5-4 h before the injection of the phlogistic agent (P < 0.01). Treatment of the animals with salbutamol (5 mg kg-1, i.p.), 0.5-24 h before pleurisy induction, did not inhibit either cell migration or fluid leakage. In this condition, a significant increase of these parameters was observed in the group pretreated with salbutamol 8-24 h before pleurisy induction (P < 0.01). 5. These results indicate that theophylline and cromolyn were able to inhibit the early (4 h) and late (48 h) phases of the inflammatory reaction induced by carrageenin in a murine model of pleurisy. Salbutamol was effective only against the early phase. The inhibitory effects of theophylline, cromolyn and salbutamol on the early phase of this inflammatory reaction were long-lasting, although a distinct profile of inhibition was observed among them. These findings confirm and extend previous results described in other models of asthma and support both clinical and experimental evidence suggesting that these anti-asthmatic agents exhibit marked anti-inflammatory properties.
Full text
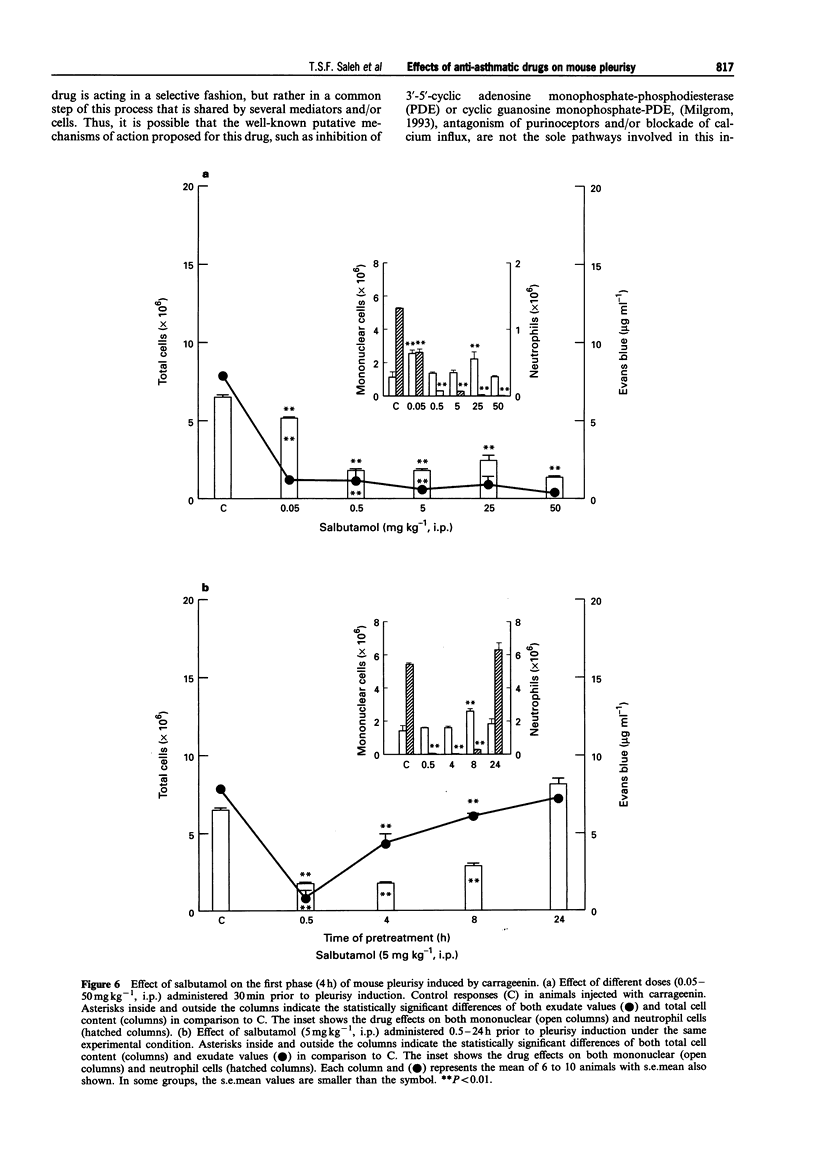
PDF
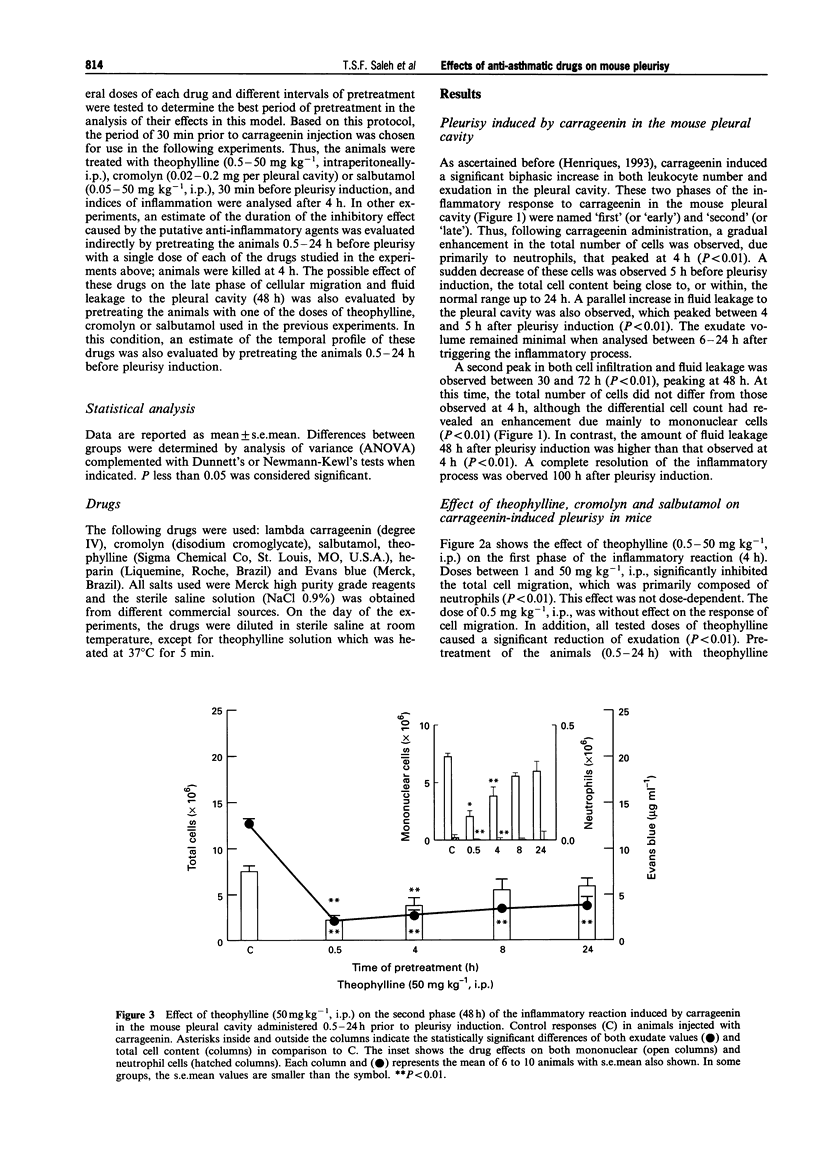
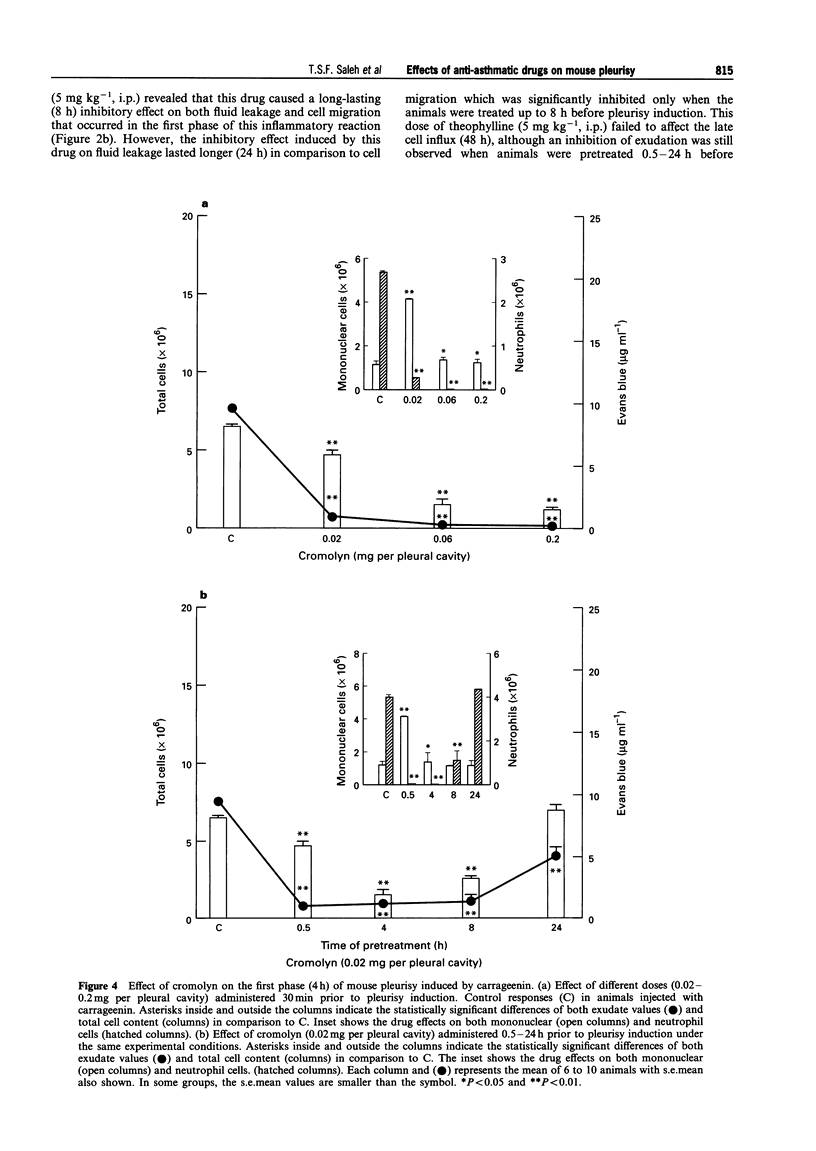
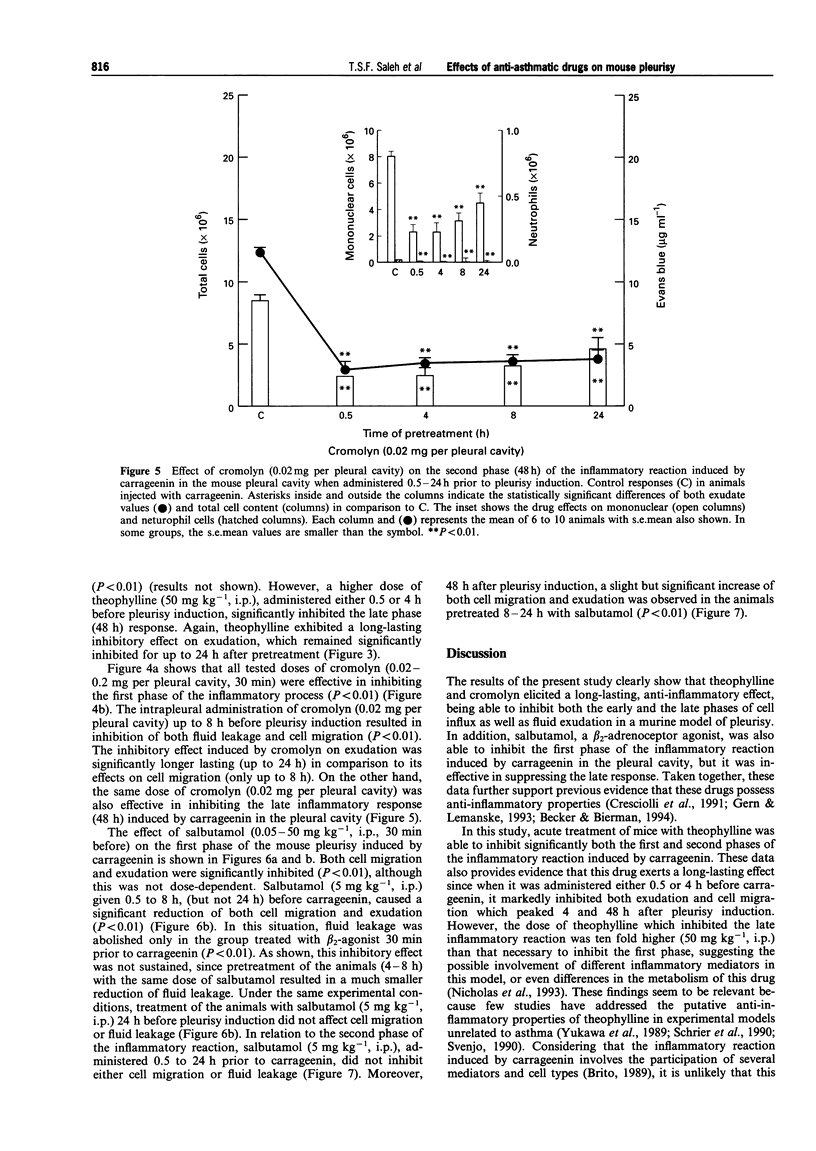
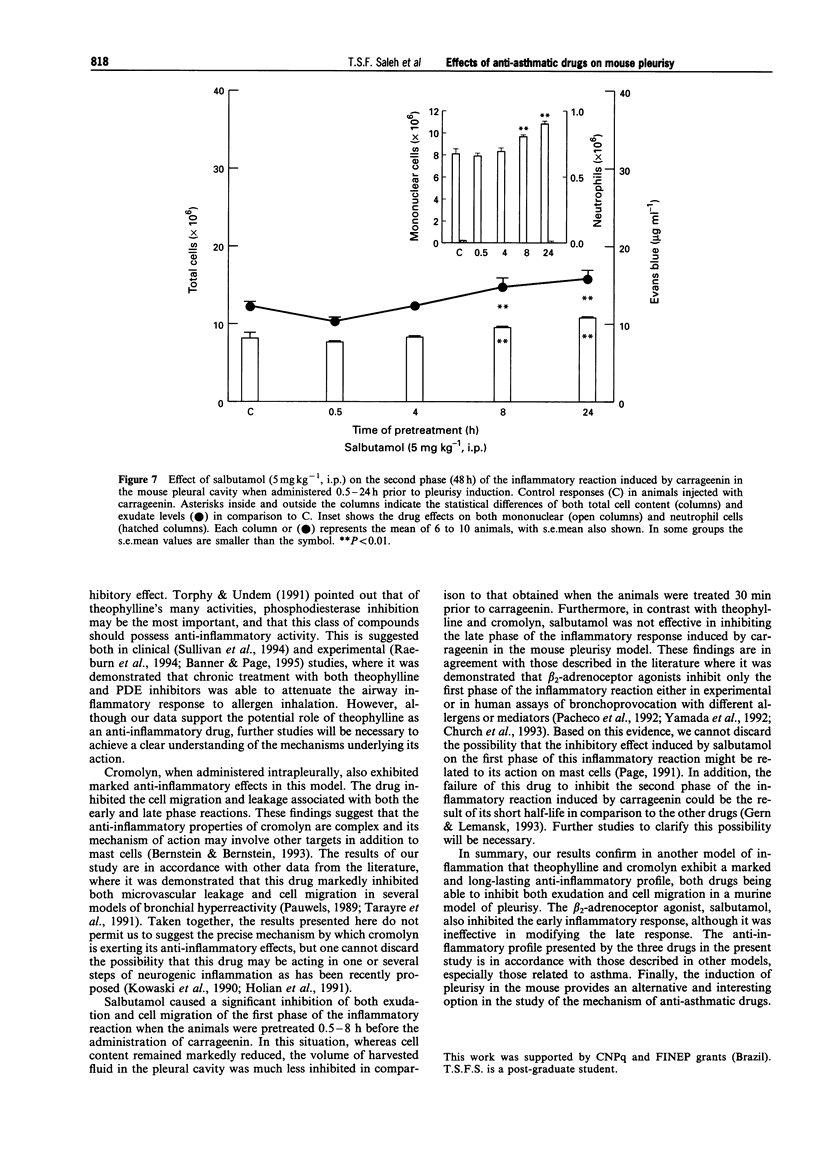

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalbers R., Smith M., Timens W. Immunohistology in bronchial asthma. Respir Med. 1993 Aug;87 (Suppl B):13–21. doi: 10.1016/0954-6111(93)90119-k. [DOI] [PubMed] [Google Scholar]
- Banner K. H., Page C. P. Acute versus chronic administration of phosphodiesterase inhibitors on allergen-induced pulmonary cell influx in sensitized guinea-pigs. Br J Pharmacol. 1995 Jan;114(1):93–98. doi: 10.1111/j.1476-5381.1995.tb14910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. A new approach to the treatment of asthma. N Engl J Med. 1989 Nov 30;321(22):1517–1527. doi: 10.1056/NEJM198911303212206. [DOI] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Church M. K., Hutson P. A., Holgate S. T. Nedocromil sodium blocks the early and late phases of allergen challenge in a guinea pig model of asthma. J Allergy Clin Immunol. 1993 Jul;92(1 Pt 2):177–182. doi: 10.1016/0091-6749(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Crescioli S., Spinazzi A., Plebani M., Pozzani M., Mapp C. E., Boschetto P., Fabbri L. M. Theophylline inhibits early and late asthmatic reactions induced by allergens in asthmatic subjects. Ann Allergy. 1991 Mar;66(3):245–251. [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Pharmacologic control of plasma exudation into tracheobronchial airways. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1008–1014. doi: 10.1164/ajrccm/143.5_Pt_1.1008. [DOI] [PubMed] [Google Scholar]
- Hargreave F. E., Ryan G., Thomson N. C., O'Byrne P. M., Latimer K., Juniper E. F., Dolovich J. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol. 1981 Nov;68(5):347–355. doi: 10.1016/0091-6749(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Henriques M. G., Rae G. A., Cordeiro R. S., Williams T. J. Endothelin-1 inhibits PAF-induced paw oedema and pleurisy in the mouse. Br J Pharmacol. 1992 Jul;106(3):579–582. doi: 10.1111/j.1476-5381.1992.tb14378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford N., Black P., Couch R., Kennedy J., Briant R. Theophylline target concentration in severe airways obstruction - 10 or 20 mg/L? A randomised concentration-controlled trial. Clin Pharmacokinet. 1993 Dec;25(6):495–505. doi: 10.2165/00003088-199325060-00007. [DOI] [PubMed] [Google Scholar]
- Holian A., Hamilton R., Scheule R. K. Mechanistic aspects of cromolyn sodium action on the alveolar macrophage: inhibition of stimulation by soluble agonists. Agents Actions. 1991 Jul;33(3-4):318–325. doi: 10.1007/BF01986580. [DOI] [PubMed] [Google Scholar]
- Kemp J. P. Approaches to asthma management. Realities and recommendations. Arch Intern Med. 1993 Apr 12;153(7):805–812. [PubMed] [Google Scholar]
- Kowalski M. L., Sliwinska-Kowalska M., Kaliner M. A. Neurogenic inflammation, vascular permeability, and mast cells. II. Additional evidence indicating that mast cells are not involved in neurogenic inflammation. J Immunol. 1990 Aug 15;145(4):1214–1221. [PubMed] [Google Scholar]
- Lo T. N., Almeida A. P., Beaven M. A. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther. 1982 Apr;221(1):261–267. [PubMed] [Google Scholar]
- Morley J. K+ channel openers and suppression of airway hyperreactivity. Trends Pharmacol Sci. 1994 Dec;15(12):463–468. doi: 10.1016/0165-6147(94)90060-4. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich J., Hargreave F. E. Late asthmatic responses. Am Rev Respir Dis. 1987 Sep;136(3):740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- Okayama Y., Church M. K. Comparison of the modulatory effect of ketotifen, sodium cromoglycate, procaterol and salbutamol in human skin, lung and tonsil mast cells. Int Arch Allergy Immunol. 1992;97(3):216–225. doi: 10.1159/000236122. [DOI] [PubMed] [Google Scholar]
- Pacheco Y., Hosni R., Chabannes B., Gormand F., Moliere P., Grosclaude M., Piperno D., Lagarde M., Perrin-Fayolle M. Leukotriene B4 level in stimulated blood neutrophils and alveolar macrophages from healthy and asthmatic subjects. Effect of beta-2 agonist therapy. Eur J Clin Invest. 1992 Nov;22(11):732–739. doi: 10.1111/j.1365-2362.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Page C. P. One explanation of the asthma paradox: inhibition of natural anti-inflammatory mechanism by beta 2-agonists. Lancet. 1991 Mar 23;337(8743):717–720. doi: 10.1016/0140-6736(91)90289-2. [DOI] [PubMed] [Google Scholar]
- Paggiaro P. L., Dente F. L., Vagaggini B., Bacci E., Talini D., Testi R., Mapp C. E., Fabbri L. M., Giuntini C. Salbutamol plus beclomethasone dipropionate, but not salbutamol alone, completely prevent early and late asthmatic responses to allergen. Respir Med. 1991 Sep;85(5):401–406. doi: 10.1016/s0954-6111(06)80185-x. [DOI] [PubMed] [Google Scholar]
- Pauwels R. A. New aspects of the therapeutic potential of theophylline in asthma. J Allergy Clin Immunol. 1989 Feb;83(2 Pt 2):548–553. doi: 10.1016/0091-6749(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Raeburn D., Underwood S. L., Lewis S. A., Woodman V. R., Battram C. H., Tomkinson A., Sharma S., Jordan R., Souness J. E., Webber S. E. Anti-inflammatory and bronchodilator properties of RP 73401, a novel and selective phosphodiesterase type IV inhibitor. Br J Pharmacol. 1994 Dec;113(4):1423–1431. doi: 10.1111/j.1476-5381.1994.tb17156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier D. J., Lesch M. E., Wright C. D., Gilbertsen R. B. The antiinflammatory effects of adenosine receptor agonists on the carrageenan-induced pleural inflammatory response in rats. J Immunol. 1990 Sep 15;145(6):1874–1879. [PubMed] [Google Scholar]
- Smith H. Asthma, inflammation, eosinophils and bronchial hyperresponsiveness. Clin Exp Allergy. 1992 Feb;22(2):187–197. doi: 10.1111/j.1365-2222.1992.tb03072.x. [DOI] [PubMed] [Google Scholar]
- Spatafora M., Chiappara G., Merendino A. M., D'Amico D., Bellia V., Bonsignore G. Theophylline suppresses the release of tumour necrosis factor-alpha by blood monocytes and alveolar macrophages. Eur Respir J. 1994 Feb;7(2):223–228. doi: 10.1183/09031936.94.07020223. [DOI] [PubMed] [Google Scholar]
- Sullivan P., Bekir S., Jaffar Z., Page C., Jeffery P., Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994 Apr 23;343(8904):1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Tainsh K. R., Lau H. Y., Liu W. L., Pearce F. L. The human skin mast cell: a comparison with the human lung cell and a novel mast cell type, the uterine mast cell. Agents Actions. 1991 May;33(1-2):16–19. doi: 10.1007/BF01993115. [DOI] [PubMed] [Google Scholar]
- Tarayre J. P., Aliaga M., Barbara M., Tisseyre N., Vieu S., Tisne-Versailles J. Pharmacological modulation of a model of bronchial inflammation after aerosol-induced active anaphylactic shock in conscious guinea pigs. Int J Immunopharmacol. 1991;13(4):349–356. doi: 10.1016/0192-0561(91)90004-q. [DOI] [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Kadowaki S., Umezu K. Development of an animal model of late asthmatic response in guinea pigs and effects of anti-asthmatic drugs. Prostaglandins. 1992 Jun;43(6):507–521. doi: 10.1016/0090-6980(92)90111-6. [DOI] [PubMed] [Google Scholar]
- Yukawa T., Kroegel C., Chanez P., Dent G., Ukena D., Chung K. F., Barnes P. J. Effect of theophylline and adenosine on eosinophil function. Am Rev Respir Dis. 1989 Aug;140(2):327–333. doi: 10.1164/ajrccm/140.2.327. [DOI] [PubMed] [Google Scholar]


