Abstract
Objective:
To report the presence of microscopic neoplasms of the testis in men with anti-Ma2-associated encephalitis (Ma2-encephalitis) and to discuss the clinical implications.
Methods:
Orchiectomy specimens were examined using immunohistochemistry with Ma2 and Oct4 antibodies.
Results:
Among 25 patients with Ma2-encephalitis younger than 50 years, 19 had germ-cell tumors, and 6 had no evidence of cancer. These 6 patients underwent orchiectomy because they fulfilled five criteria: 1) demonstration of anti-Ma2 antibodies in association with MRI or clinical features compatible with Ma2-encephalitis, 2) life-threatening or progressive neurologic deficits, 3) age < 50 years, 4) absence of other tumors, and 5) new testicular enlargement or risk factors for germ-cell tumors, mainly cryptorchidism or ultrasound evidence of testicular microcalcifications. All orchiectomy specimens showed intratubular-germ cell neoplasms unclassified type (IGCNU) and other abnormalities including microcalcifications, atrophy, fibrosis, inflammatory infiltrates, or hypospermatogenesis. Ma2 was expressed by neoplastic cells in three of three patients examined. Even though most patients had severe neurologic deficits at the time of orchiectomy (median progression of symptoms, 10 months), 4 had partial improvement and prolonged stabilization (8 to 84 months, median 22.5 months) and two did not improve after the procedure.
Conclusions:
In young men with Ma2-encephalitis, 1) the disorder should be attributed to a germ-cell neoplasm of the testis unless another Ma2-expressing tumor is found, 2) negative tumor markers, ultrasound, body CT, or PET do not exclude an intratubular germ-cell neoplasm of the testis, and 3) if no tumor is found, the presence of the five indicated criteria should prompt consideration of orchiectomy.
Tumors involved in paraneoplastic neurologic disorders are usually small, confined to a specific organ, or detectable at the organ-draining lymph nodes.1,2 Recent studies show that the use of CT and FDG-PET uncovers most of these tumors at symptom presentation or within the first year of the neurologic disorder.3 Among all paraneoplastic disorders, the anti-Ma2 immune response is the most specific for limbic, diencephalic, or upper brainstem encephalitis.4 In young men this disorder associates with testicular tumors, while in older men or women other tumors are involved. Of 25 men with anti-Ma2-associated encephalitis younger than 50 years, 19 had germ-cell tumors (18 in testis). The current study focuses on the remaining 6 patients whose tumors were not found by multiple ancillary tests or even at initial evaluation of the orchiectomy specimen. Eventually all 6 patients were found to have a microscopic intratubular germ-cell neoplasm unclassified type (IGCNU), a common precursor of most testicular cancers that takes approximately 5 years to become invasive.5
Methods
Tissues and antibodies
The 6 patients were seen by the authors and form part of a series of 46 patients with anti-Ma2-associated encephalitis (appendix E-1 on the Neurology Web site at www.neurology.org).6 One of the 6 patients has been reported in detail.7 Patients or family members consented for antibody and tumor studies. These studies were approved by the University of Pennsylvania Institutional Review Board. Tumor tissue was available from all 6 patients: 1 frozen and 5 embedded in paraffin. Detection of anti-Ma1 and Ma2 antibodies was performed using immunoblot of recombinant proteins, as reported.8 Oct4 affinity-purified goat antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used to re-examine orchiectomy specimens for tumor cells. IgG containing anti-Ma2 antibodies isolated from patients' sera and labeled with biotin was used to determine the expression of Ma2 in tumor cells.9
Immunohistochemistry
Paraffin-embedded tissue was deparaffinized and the antigens retrieved, as reported.10 Tissue sections were serially incubated with 0.3% H2O2 for 20 minutes, 10% goat serum for 1 hour, biotinylated patients' IgG (80 μg/mL) or anti-Oct4 (1:200) overnight at 4 °C. Sections incubated with anti-Oct4 were subsequently incubated with biotinylated horse anti-goat IgG (Vector, Burlingame, CA). For all sections the reactivity was developed with the avidin-biotin peroxidase method. Sections incubated with biotinylated normal human IgG or normal goat serum served as controls.
Double immunolabeling with Ma2 and Oct4 antibodies was performed using the appropriate Alexa Fluor secondary antibodies (Molecular Probes, OR). Images were photographed under a Zeiss fluorescence microscope using Axiovision software.
Results
General clinical features
The 6 patients developed short-term memory deficits due to limbic dysfunction, and additional symptoms that resulted from involvement of the hypothalamus-diencephalon, brainstem, pallidum (severe hypokinesis), or cerebellar pathways (mild, asymmetric ataxia) (table and appendix E-1). In 5 cases symptoms correlated with the MRI findings (figure 1) and in 1 the initial MRI was normal and no further studies were obtained due to severe obesity caused by hypothalamic dysfunction. CSF studies were abnormal in 4 of 5 patients examined (2 pleocytosis, 3 increased proteins, 2 intrathecal synthesis of IgG; data not shown). All patients had Ma2 antibodies in serum and CSF (available in 5); none had Ma1 antibodies.
Table.
Clinical features, orchiectomy findings, and neurologic outcome
| Patient (age, y) |
Duration of symptoms* |
Syndrome | MRI | Reasons for orchiectomy (US findings) |
Initial pathology report | Final diagnosis |
Response to treatment: MRS† at presentation (after orchiectomy); follow-up |
|---|---|---|---|---|---|---|---|
| 1 (35) | 6 | Hypothalamic, brainstem, limbic | Temporal lobes, hypothalamus, brainstem | 5 criteria‡; (right TM) | No tumor; TM | IGCNU | Partial: 5 (4); ½ y |
| 2 (36) | 3 | Limbic | Bilateral temporal lobes | 5 criteria; history of cryptorchidism; (TM, right fibrosis vs tumor) | No tumor; dense collagen; atrophic tubules; TM; Sertoli cell nodules | IGCNU | Stable: 4; 3 ½ y |
| 3 (40) | 8 | Hypokinesis, atypical parkinsonism, gaze paresis; limbic; dysautonomia, intermittent dystonia | Temporal lobes, medial thalami, pulvinars, pallidi, brainstem | 5 criteria; (initially normal; follow-up showed bilateral TM) | Bilateral orchiectomy: no tumor; fibrous scar; atrophic tubules; TM; infiltrates of lymphocytes and macrophages; hypospermatogenesis | Bilateral IGCNU | Stable: 5; died 7.5 mo after orchiectomy |
| 4 (35) | 16 | Limbic, hypothalamic, cataplexy, EDS | Bilateral temporal lobes | 5 criteria; mildly enlarged right testis (right hydrocele, epididymitis) | No tumor; occasional inflammatory infiltrates | IGCNU | Partial: 4 (2); 7 y |
| 5 (26) | 12 | Limbic, brainstem, hypothalamic, cataplexy, EDS | Normal | 5 criteria; (initially normal; follow-up showed right TM) | IGCNU; fibrous scar; TM; atrophic and obliterated tubules; infiltrates of lymphocytes and plasma cells; hypospermatogenesis | IGCNU | Partial: 4 (3); died of PE 15 mo after diagnosis |
| 6 (28) | 32 | Limbic, asymmetric ataxia | Mild cerebellar atrophy | 5 criteria; enlarged right testis (3 mm abnormality in the left testis) | Right orchiectomy: no tumor; focal TM; left orchiectomy: no tumor; atrophic and obliterated tubules; fibrosis, TM | Bilateral IGCNU | Partial: 3 (2); 8 mo |
Duration of symptoms: time from symptom presentation to orchiectomy (months).
Five criteria: 1) demonstration of anti-Ma2 antibodies in association with MRI or clinical features compatible with Ma2-encephalitis, 2) life-threatening or progressive neurologic deficits, 3) age <50 years, 4) absence of other tumors, and 5) new testicular enlargement or risk factors for germ-cell tumors (cryptorchidism or US evidence of TM).
Modified Rankin Scale.29
US = ultrasound; TM = testicular microcalcifications; IGCNU = intratubular germ-cell neoplasm unclassified type; EDS = excessive day time sleepiness; PE = pulmonary embolism.
Figure 1.
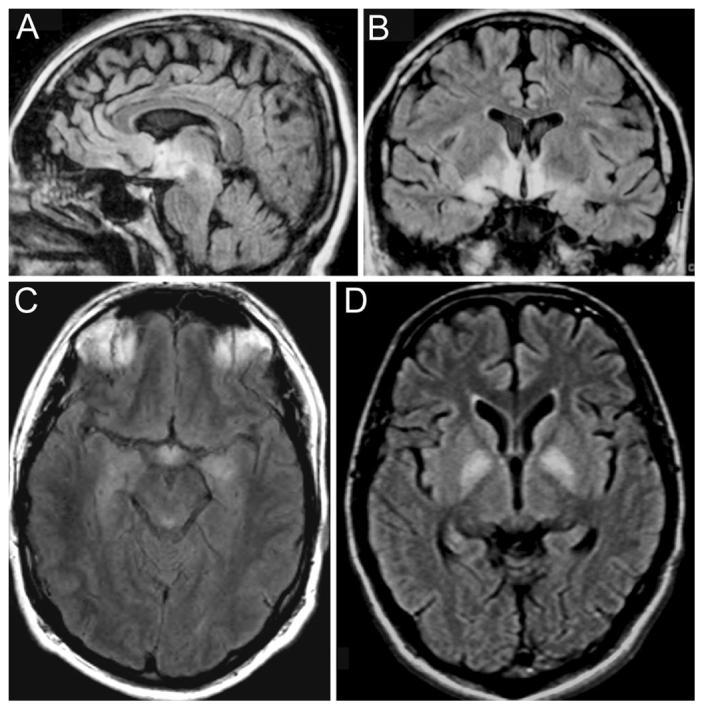
MRI of patients with anti-Ma2-associated encephalitis. The images demonstrate the fluid-attenuated inversion-recovery (FLAIR) MRI sequences of three patients. The MRI of Patient 1 (A and B) shows the areas more frequently involved in patients with anti-Ma2-associated encephalitis, including medial temporal lobes, hypothalamus, and upper brainstem. C corresponds to Patient 2 and shows involvement of the medial temporal lobes and hypothalamus. D corresponds to Patient 3 whose symptoms of severe hypokinesis correlated with bilateral involvement of the pallidum; this patient also had FLAIR hyperintensities involving the medial thalami, pulvinars, hippocampi, and upper brainstem (not shown here).
Cancer screening
All patients underwent CT of the chest, abdomen, and pelvis, which were negative in five and in one showed thickening of the seminal vesicle of unclear etiology. Body FDG-PET was obtained in two patients (Cases 2 and 3) and found to be normal. Tumor markers including α-fetoprotein and β-HCG were negative in five patients; one patient had mild transient elevation of β-HCG that returned spontaneously to normal during the cancer screening. Testicular ultrasound was considered negative for the presence of a tumor in five patients and showed abnormalities compatible with fibrosis or tumor in a patient with history of cryptorchidism (table). In one patient with mild right testicular enlargement, the ultrasound revealed a unilateral hydrocele and findings suggesting epididymitis. Two patients had unilateral microcalcifications, and another two patients developed uni- and bilateral microcalcifications that were not present at initial ultrasound evaluation.
Orchiectomy: Reasons and effects on neurologic symptoms
All six patients underwent orchiectomy for the following reasons: 1) demonstration of anti-Ma2 antibodies in association with MRI or clinical features compatible with Ma2-encephalitis,4 2) life-threatening or progressive neurologic deficits, 3) age <50 years, 4) absence of other tumors, and 5) new testicular enlargement or risk factors for germ-cell tumors (microcalcifications, cryptorchidism), as indicated above.
Four patients had unilateral orchiectomy. One patient had bilateral radical orchiectomy based on the rapid progression of severe deficits, development of microcalcifications in both testis, and the known occurrence of bilateral germ-cell neoplasms. Another patient had a right radical orchiectomy based on the presence of an enlarged and painful testis that at initial pathologic evaluation showed no tumor, and he elected to have a partial left orchiectomy a few weeks later.
By the time of the orchiectomy five patients had severe neurologic deficits and one was moderately affected (table). After orchiectomy, four patients had mild improvement and prolonged stabilization (8 to 84 months, median 22.5 months); three are alive and one died of unrelated causes 15 months after orchiectomy. These patients underwent orchiectomy 6 to 32 months after symptom development (median 14 months) and the improvement occurred within 3 weeks of orchiectomy. The symptoms that showed early improvement were seizures (3 patients), narcolepsycataplexy,2 and episodes of hyperthermia.1 In contrast, two patients did not improve; compared with the previous group they underwent orchiectomy relatively early (3 and 8 months after symptom development) but were severely affected. One (Case 3) was bedridden with severe rigidity and hypokinesis when a bilateral orchiectomy was performed; his symptoms stabilized but he died 7.5 months later of systemic complications. The other patient (Case 2) had a unilateral orchiectomy and has remained with severe deficits and high serum and CSF titers of Ma2 antibodies for 3½ years.
Immunopathologic findings
In one patient the initial pathologic study revealed an intratubular germ-cell neoplasm unclassified type (IGCNU) with in situ spread into the rete testis (figure 2A). In the other five patients (including the two cases with bilateral orchiectomy) the initial evaluation was negative for the presence of a tumor but identified several pathologic abnormalities (table). In four of these five patients the presence of an IGCNU was subsequently demonstrated using routine pathologic studies upon request of reassessment; in all instances the tumor cells expressed placental alkaline phosphatase (PLAP) and Oct4 (figures 2 and 3). In the other patient, a few foci of Oct4 positive cells were identified in a retrospective evaluation of a frozen block of tissue: of 250 consecutive sections only 9 had a few remaining tubules with Oct4 positive cells in association with extensive fibrosis (data not shown).
Figure 2.
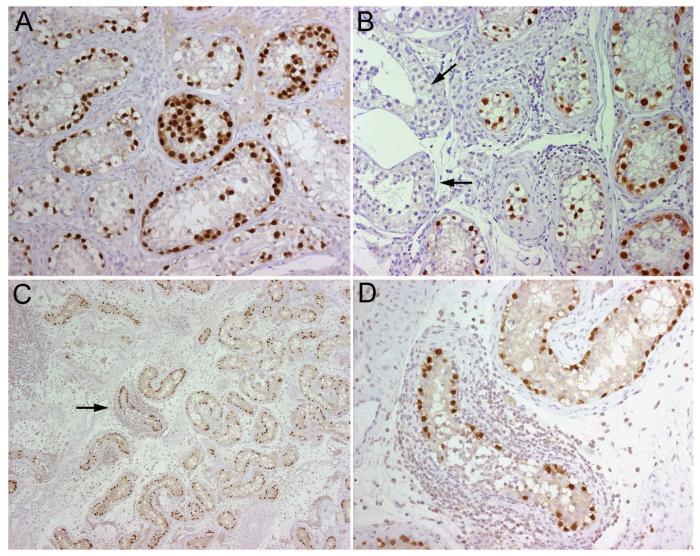
Demonstration of germ-cell tumors with Oct4 antibody. All panels correspond to paraffin embedded orchiectomy specimens immunolabeled with anti-Oct4 antibody (A, Patient 5; B, Patient 1; C and D, Patient 3). Note the intense immunolabeling of the neoplastic cells (brown staining) but lack of reactivity of the epithelium of normal tubules (arrows in B). C shows Oct4-expressing cells detectable at low magnification; there are several inflammatory infiltrates, one of them surrounds a tubule with neoplastic cells (arrow) that is shown amplified in D. All sections counterstained with hematoxylin. A, B, D, x200; C, x5.
Figure 3.
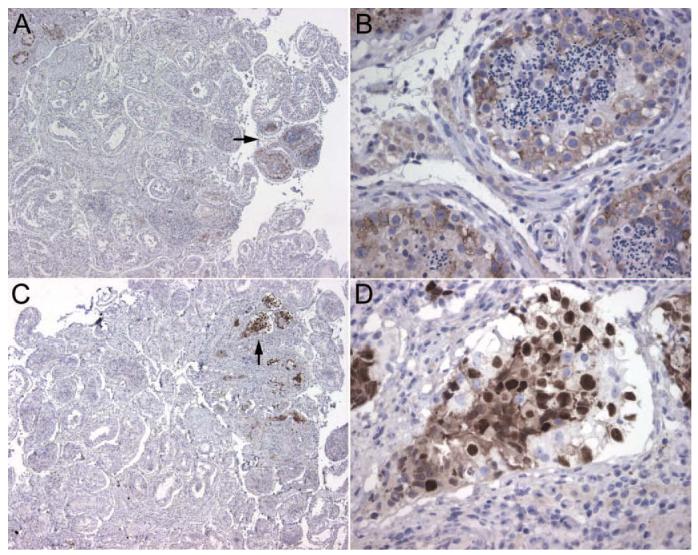
Demonstration of small foci of neoplastic cells using PLAP and Oct4 antibodies. All panels correspond to paraffin embedded orchiectomy specimens from Patient 6. In A, the neoplastic cells are demonstrated with PLAP staining; the arrow indicates a focus of neoplastic cells that is shown at higher magnification in B. In C, another focus of neoplastic cells is demonstrated with Oct4 staining; the arrow points to the area amplified in D. Note that the Oct4 staining is more intense than PLAP and easier to detect at low magnification. All sections counterstained with hematoxylin. A, C, x5; B, D, x400.
Double labeling studies with Oct4 and Ma2 antibodies were performed in three tumors. In all instances, Ma2 was co-expressed with Oct4, indicating that Ma2 was present in the neoplastic but not the normal cells (figure 4).
Figure 4.
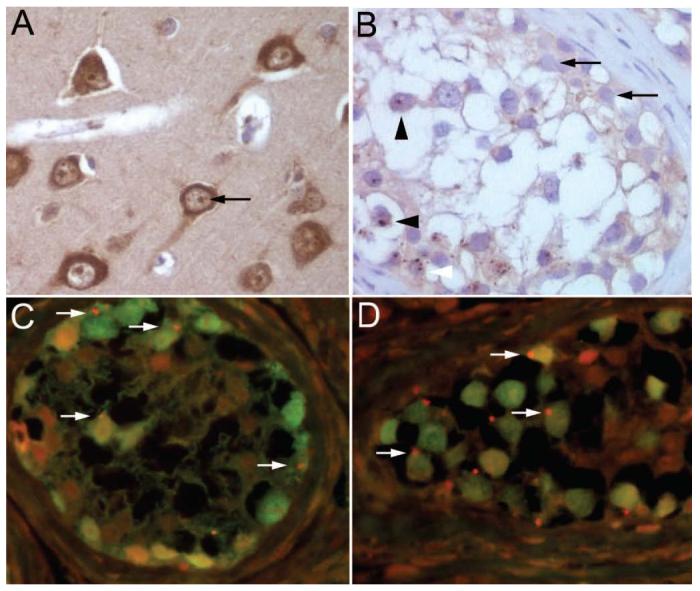
Expression of Ma2 by intratubular germ-cell neoplasms. (A) Paraffin section of human hippocampus obtained from autopsy of a neurologically normal individual, immunolabeled with biotinylated IgG containing anti-Ma2 antibodies. Note that in normal brain Ma2 is expressed in the cytoplasm and nucleus of neurons with a pattern of dot-like nuclear reactivity resembling speckles (arrow). (B) Paraffin section of a seminiferous tubule of Patient 5 immunolabeled with biotinylated IgG containing anti-Ma2 antibodies. Ma2 protein is expressed as dot-like speckles by the neoplastic germ-cells (arrow heads), but not by normal epithelium (arrows). Confirmation of Ma2 expression by neoplastic cells was also obtained with a double labeling immunofluorescence method shown in C, D. (C, D) Sections of seminiferous tubules of Patients 3 (C) and 5 (D) immunolabeled with anti-Oct4 (green) and biotinylated IgG containing anti-Ma2 antibodies (red). The expression of Ma2 (red dots) by several neoplastic cells (green nuclei) is indicated with arrows; some cells also show mild diffuse coexpression of Ma2 (yellow). A, B, avidin-biotin-peroxidase method; counterstained with hematoxylin, x400. C, D, double labeling immunofluorescence method, x400.
Discussion
We report six patients with progressive neurologic deficits in whom the recognition of the disorder as paraneoplastic anti-Ma2-associated encephalitis led to uni- or bilateral orchiectomy, exposing in all instances a tumor at the microscopic or preinvasive stage. More importantly, despite the severity of the deficits, four patients had mild neurologic improvement followed by prolonged stabilization after tumor resection.
In most respects the neurologic and MRI features of these patients are similar to those previously reported in a series of 38 patients with anti-Ma2-associated encephalitis.4 This immunity is notable because it associates with dysfunction of the limbic system, diencephalon, or upper brainstem in 95% of the patients, and has not been encountered in patients without paraneoplastic disease.8,11 In an updated review of our experience with 46 patients, 25 were younger than 50 years (including the 6 cases reported here) and all were men with germ-cell tumors. Among the group of patients older than 50 years (64% women) all had tumors other than germ-cell neoplasms, with non-small cell lung cancer the most common tumor (52%; appendix E-1).
Demonstration of the tumor is particularly important in patients with anti-Ma2-associated encephalitis because the disorder responds to treatment in about a third of patients.4,12 The features that associate with improvement include limited involvement of the nervous system, absence of Ma1 antibodies, and prompt treatment of the tumor, which with a few exceptions13 is a testicular germ-cell neoplasm.4 Our six patients had some of these features but the tumor could not be detected on clinical grounds or at initial pathologic evaluation and this resulted in important diagnostic delays (median 10 months). After extensive discussion with the patients and families, the decision to proceed to orchiectomy was based on the above indicated reasons along with findings that assisted in deciding on the side of the procedure, such as the presence of microcalcifications or a history of cryptorchidism.
The difficulty in demonstrating the tumor extended to the pathologic examination. At initial evaluation of the orchiectomy, neoplastic cells were identified in only one patient. In four patients the tumor was found at reassessment upon request of the neurologist and in one in a retrospective review of archived tissue (discussed below). In all instances, the tumor was a carcinoma in situ or IGCNU.14 Two problems in the demonstration of this type of tumor are the microscopic size and the relative normal appearance of the cells that may escape detection unless specific markers on numerous sections are used.
Placental-like alkaline phosphatase (PLAP) is the standard immunohistochemical marker used to identify germ-cell tumors.14,15 However, it is not totally specific because non-neoplastic spermatocytes may show PLAP reactivity and it associates with background staining. Recent studies indicate that Oct4, a transcription factor that regulates initiation, maintenance, and differentiation of pluripotent germline cells during normal development, is a highly sensitive and specific marker of germ-cell tumors, including IGCNU.16,17 Our patients' orchiectomy specimens were re-examined for expression of Oct4 and in all instances it was present in the neoplastic cells. Furthermore, the intense reactivity with the nuclei of the cells and absence of background in the peroxidase staining allowed detection of the tumor even at low magnification. One of the six patients' specimens had been PLAP negative and he remained without a tumor diagnosis until the tissue was re-examined 4 years later, revealing foci of Oct4 positive cells.
IGCNU is the precursor of invasive germ-cell tumors and is found in essentially every case of testicular germ-cell tumor.14 In an autopsy study of 1,388 presumably healthy men (median age 33 years) who died unexpectedly, only 6 (0.43%) had IGCNU.18 About 50% of patients with a biopsy positive for IGCNU develop invasive germ-cell tumors within 5 years, and only a small fraction remain tumor free by 7 years.5 At least 6% of patients with testicular tumors have contralateral IGCNU; this estimate is much higher if the contralateral testis is atrophic (<20 to 34%).19 Development of testicular microcalcification (TM) has a strong association with tumors.20 TM is believed to result from sloughing of damaged cells into the tubule resulting in degeneration possibly associated with an immunologic process, and precipitation of a glycoprotein that forms microliths.21,22 When TM is diagnosed, a coexistent tumor is present in 30 to 40% of patients.23 In agreement with these studies, five of our patients had TM, four identified by ultrasound and one at orchiectomy.
The exact mechanism whereby a microscopic tumor confined to the testis (an immunoprivileged organ) triggers the anti-Ma2 immune response is unknown. There is experimental evidence that sub-populations of testicular macrophages are efficient antigen presenting cells in humoral- and cell-mediated immune responses,24 and that damaged seminiferous tubules express increased amounts of cytokines promoting immune responses.25 Our study shows that the tumor cells of the patients expressed Ma2, a protein that is normally only expressed in neurons. This finding along with the accompanying pathologic abnormalities (microcalcification, inflammatory infiltrates, or macrophages) suggests the presence of a microenvironment that could have set off the anti-Ma2 immune response at the tumor site.
The effects of orchiectomy on neurologic outcome appeared to be dependent on the severity rather than duration of symptoms. Among the four patients who had partial improvement, the median duration of symptoms pre-orchiectomy was 14 months. Interestingly, the one with the longest clinical course (32 months) was the less severely affected (i.e., able to travel by himself from another state). In contrast, the two patients without improvement had a relatively shorter duration of symptoms (3 and 8 months), but one was bedridden due to his rigidity and immobility, and the other has remained with amnesia, bursts of aggression, hyperphagia, and high Ma2 antibodies for 3½ years. The persistence of high antibody titers (serum 1:2,000,000) and the fact that the two patients with bilateral orchiectomy had bilateral tumors raises the question of whether this patient may have a contralateral tumor. No follow-up antibody titers are available from the other patients, but there is recent evidence that Ma2 antibody titers may correlate with the clinical course.13
The current and previous studies indicate that detection of Ma2 antibodies in young men strongly associates with testicular tumors.4,26 Of 33 men with anti-Ma2-associated encephalitis that we have studied, all patients younger than 50 years and none above this age (58 to 71 years, median 67) had germ-cell tumors. It is known that non-seminomatous germ-cell tumors peak in the third decade of life and seminomas in the fourth decade.14 Therefore, although recommendations based on patient's age should be taken cautiously, any man younger than 50 years with progressive deficits related to anti-Ma2-associated encephalitis should be considered to have a germ-cell tumor of the testis unless another Ma2-expressing tumor is found. In these patients scrotal ultrasound and serologic testing for β-HCG and α-fetoprotein (often negative in seminomas, IGCNU, and teratomas) are mandatory. If the tumor is not found, a history of cryptorchidism and atrophy, and particularly the detection of TM, should lead to consideration of orchiectomy. If the ultrasound is normal and there are no risk factors for a tumor, and the patient develops progressive or life-threatening neurologic complications, a blind unilateral orchiectomy should be considered and if negative, a partial or total contralateral orchiectomy may be necessary. One can argue for a multiple site testicular biopsy but false negatives do occur,27,28 and we suspect that it would have been negative in our patients whose microscopic tumors were even missed at initial histopathologic evaluation. The importance of the antibody titers in this decision plan should be clarified in future studies.
Acknowledgment
The authors thank Dr. J.A. Zabala for providing clinical information.
Footnotes
Supported in part by RO1CA089054 (J.D.).
Disclosure: The authors report no conflicts of interest.
An abstract of this article was presented at the 58th annual meeting of the American Academy of Neurology; San Diego, CA; April 5, 2006. Video clips of the presentation and commentary by Dr. K. Jaeckle are available at the AAN Web site: http://www.marathonmultimedia.com/aan_library/master2.php?ud-56136; “view sessions.”
References
- 1.Graus F, Dalmau J, Rene R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–2872. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 2.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 3.Younes-Mhenni S, Janier MF, Cinotti L, et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain. 2004;127:2331–2338. doi: 10.1093/brain/awh247. [DOI] [PubMed] [Google Scholar]
- 4.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 5.Montironi R. Intratubular germ cell neoplasia of the testis: testicular intraepithelial neoplasia. Eur Urol. 2002;41:651–654. doi: 10.1016/s0302-2838(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 6.Mathew RM, Yamamoto T, Nakamura K, Dropcho E, Tsuji S, Dalmau J. Orchiectomy for suspected microscopic tumor in patients with paraneoplastic anti-Ma2 encephalitis. 58th annual meeting of the AAN; San Diego, CA. April 5, 2006. Abstract and video clips available at: http://www.abstracts2view.com/aan/view.php?nu=AAN06L_PL01.001; http://www.marathonmultimedia.com/aan_library/master2.php?ud=56136
- 7.Landolfi JC, Nadkarni M. Paraneoplastic limbic encephalitis and possible narcolepsy in a patient with testicular cancer: case study. Neurooncol. 2003;5:214–216. doi: 10.1215/S1152-8517-02-00046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld MR, Eichen JG, Wade DF, Posner JB, Dalmau J. Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann Neurol. 2001;50:339–348. [PubMed] [Google Scholar]
- 9.Furneaux HM, Rosenblum MK, Dalmau J, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322:1844–1851. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 10.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 11.Voltz R, Gultekin SH, Rosenfeld MR, et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med. 1999;340:1788–1795. doi: 10.1056/NEJM199906103402303. [DOI] [PubMed] [Google Scholar]
- 12.Waragai M, Chiba A, Uchibori A, Fukushima T, Anno M, Tanaka K. Anti-Ma2 associated paraneoplastic neurological syndrome presenting as encephalitis and progressive muscular atrophy. J Neurol Neurosurg Psychiatry. 2006;77:111–113. doi: 10.1136/jnnp.2005.068775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal DT, Salzman KL, Digre KB, Jensen RL, Dunson WA, Dalmau J. Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis. Neurology. 2006;67:146–149. doi: 10.1212/01.wnl.0000223647.83708.20. [DOI] [PubMed] [Google Scholar]
- 14.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 15.Hattab EM, Tu PH, Wilson JD, Cheng L. OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol. 2005;29:368–371. doi: 10.1097/01.pas.0000149709.19958.a7. [DOI] [PubMed] [Google Scholar]
- 16.Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 17.Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28:935–940. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Linke J, Loy V, Dieckmann KP. Prevalence of testicular intraepithelial neoplasia in healthy males. J Urol. 2005;173:1577–1579. doi: 10.1097/01.ju.0000154348.68575.95. [DOI] [PubMed] [Google Scholar]
- 19.Harland SJ, Cook PA, Fossa SD, et al. Intratubular germ cell neoplasia of the contralateral testis in testicular cancer: defining a high risk group. J Urol. 1998;160:1353–1357. [PubMed] [Google Scholar]
- 20.Derogee M, Bevers RF, Prins HJ, Jonges TG, Elbers FH, Boon TA. Testicular microlithiasis, a premalignant condition: prevalence, histopathologic findings, and relation to testicular tumor. Urology. 2001;57:1133–1137. doi: 10.1016/s0090-4295(01)00957-8. [DOI] [PubMed] [Google Scholar]
- 21.Vegni-Talluri M, Bigliardi E, Vanni MG, Tota G. Testicular microliths: their origin and structure. J Urol. 1980;124:105–107. doi: 10.1016/s0022-5347(17)55318-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim B, Winter TC, III, Ryu JA. Testicular microlithiasis: clinical significance and review of the literature. Eur Radiol. 2003;13:2567–2576. doi: 10.1007/s00330-003-2014-5. [DOI] [PubMed] [Google Scholar]
- 23.Miller RL, Wissman R, White S, Ragosin R. Testicular microlithiasis: a benign condition with a malignant association. J Clin Ultrasound. 1996;24:197–202. doi: 10.1002/(SICI)1097-0096(199605)24:4<197::AID-JCU6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Bryniarski K, Szczepanik M, Maresz K, Ptak M, Ptak W. Subpopulations of mouse testicular macrophages and their immunoregulatory function. Am J Reprod Immunol. 2004;52:27–35. doi: 10.1111/j.1600-0897.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 25.Sundstrom J, Verajnkorva E, Salminen E, Pelliniemi LJ, Pollanen P. Experimental testicular teratoma promotes formation of humoral immune responses in the host testis. J Reprod Immunol. 1999;42:107–126. doi: 10.1016/s0165-0378(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 26.Castle J, Sakonju A, Dalmau J, Newman-Toker DE. Anti-Ma2-associated encephalitis with normal FDG-PET: a case of pseudo-Whipple's Disease. Nat Clin Pract Neurol. 2006;10:566–572. doi: 10.1038/ncpneuro0287. [DOI] [PubMed] [Google Scholar]
- 27.Dieckmann KP, Loy V. False-negative biopsies for the diagnosis of testicular intraepithelial neoplasia (TIN)—an update. Eur Urol. 2003;43:516–521. doi: 10.1016/s0302-2838(03)00101-5. [DOI] [PubMed] [Google Scholar]
- 28.Dieckmann KP, Pottek T, Heinemann V, Hopker WW, Loy V. Four testicular biopsies failing to detect a case of testicular intraepithelial neoplasia. Acta Oncol. 2004;43:212–214. doi: 10.1080/02841860310021716. [DOI] [PubMed] [Google Scholar]
- 29.Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]