Abstract
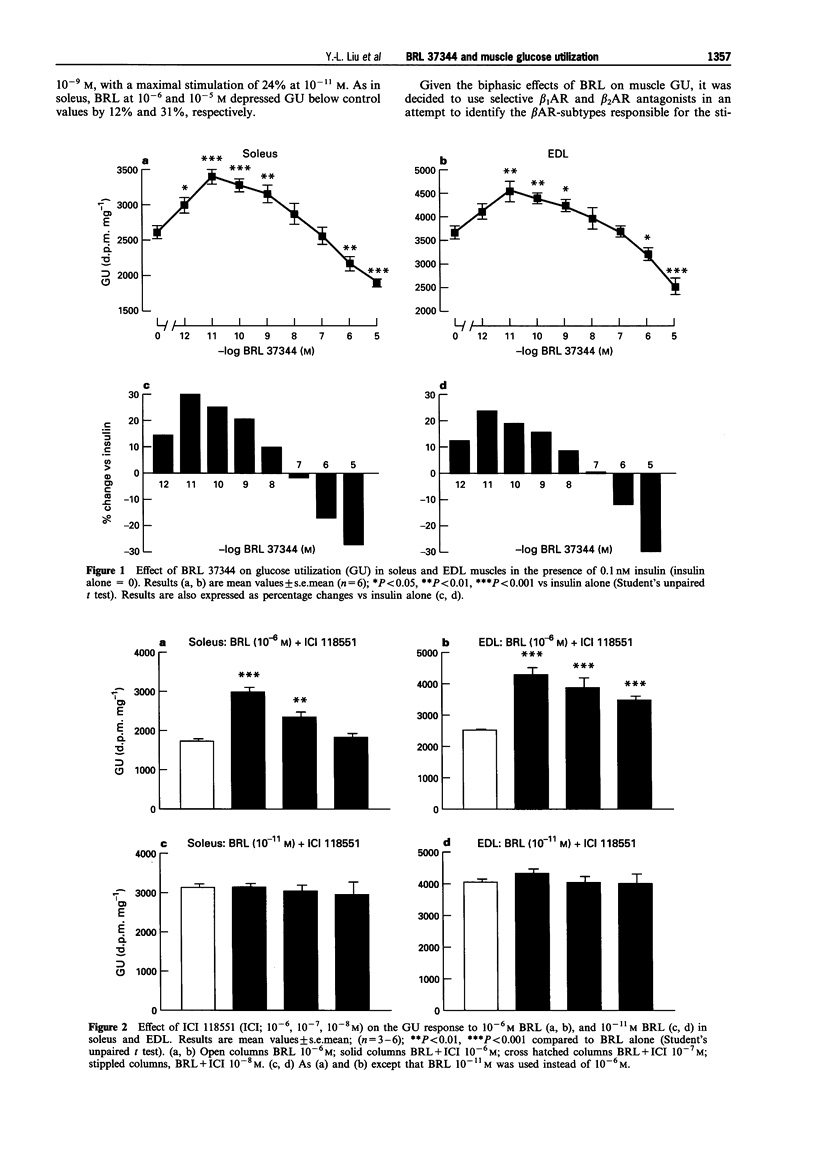
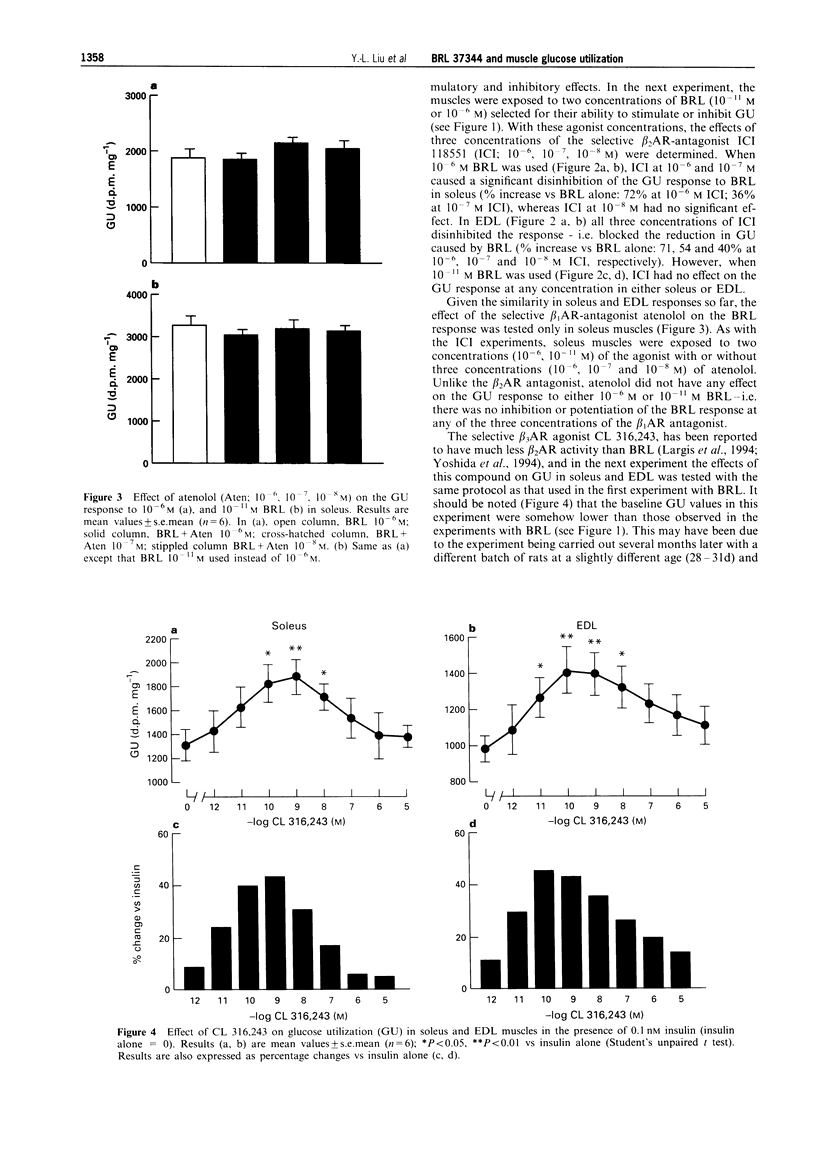
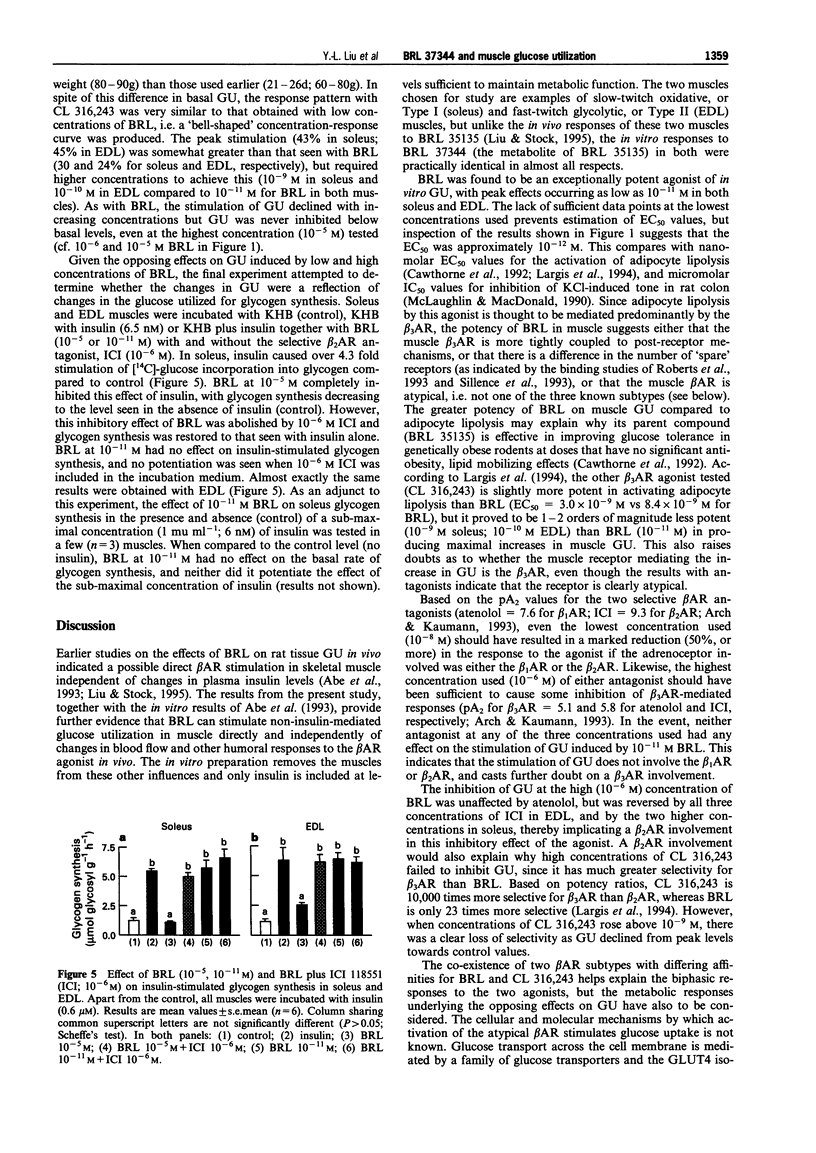
1. The effects of the selective beta 3-adrenoceptor agonist, BRL 37344 (BRL) on glucose uptake and phosphorylation (i.e. glucose utilization; GU) and glycogen synthesis in rat isolated soleus and extensor digitorium longus (EDL) muscle preparations in vitro were investigated by use of 2-deoxy-[3H]-glucose (GU) and [U-14C]-glucose (glycogen synthesis). 2. Low concentrations of BRL (10(-11)-10(-9) M) significantly increased GU, with maximal increases of 30% in soleus and 24% in EDL at 10(-11) M. Neither the selective beta 1-adrenoceptor antagonist, atenolol (10(-8)-10(-6) M), nor the selective beta 2-adrenoceptor antagonist, ICI 118551 (10(-8)-10(-6) M) had any effect on the stimulation of GU induced by 10(-11) M BRL. 3. High concentrations of BRL (10(-6)-10(-5) M) caused significant inhibition (up to 30%) of GU in both soleus and EDL muscles. The inhibition of 10(-6) M BRL was blocked completely by 10(-6) and 10(-7) M ICI 118551 in soleus, and by 10(-6)-10(-8) M ICI 118551 in EDL; atenolol (10(-8)-10(6) M) had no effect. 4. Another selective beta 3-adrenoceptor agonist, CL 316,243, also caused a significant stimulation of muscle GU, with maximal increases of 43% at 10(-9) M in soleus and 45% at 10(-10) M in EDL. The stimulation of GU declined with further increases in the concentration of CL 316,243, but no inhibition of GU was seen, even at the highest concentration (10(-5) M) tested. 5. BRL at 10(-5) M inhibited completely insulin-stimulated glycogen synthesis in both soleus and EDL, but this inhibitory effect of BRL was abolished by 10(-6) M ICI 118551. BRL at 10(-11) M (with or without 10(-6) M ICI 118551) had no effect on insulin-stimulated glycogen synthesis. 6. It is concluded that: (i) low (< nM) concentrations of BRL stimulate GU via an atypical beta-adrenoceptor that is resistant to conventional beta 1-adrenoceptor and beta 2-adrenoceptor antagonists; (ii) the stimulation of GU is negated by the activation of beta 2-adrenoceptors that occurs at higher (> nM) concentrations of BRL; (iii) inhibition of GU via beta 2-adrenoceptor activation is associated with inhibition of glycogen synthesis, possibly due to activation of glycogenolysis; (iv) the opposing effects of beta 2-adrenoceptor and atypical beta-adrenoceptor activation on GU suggest that in skeletal muscle these adrenoceptors are linked to different post-receptor pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Minokoshi Y., Shimazu T. Effect of a beta 3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J Endocrinol. 1993 Dec;139(3):479–486. doi: 10.1677/joe.0.1390479. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Kaumann A. J. Beta 3 and atypical beta-adrenoceptors. Med Res Rev. 1993 Nov;13(6):663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Steinberg H., Brechtel G., Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol. 1994 Feb;266(2 Pt 1):E248–E253. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- Berlan M., Galitzky J., Bousquet-Melou A., Lafontan M., Montastruc J. L. Beta-3 adrenoceptor-mediated increase in cutaneous blood flow in the dog. J Pharmacol Exp Ther. 1994 Mar;268(3):1444–1451. [PubMed] [Google Scholar]
- Bonen A., Clark M. G., Henriksen E. J. Experimental approaches in muscle metabolism: hindlimb perfusion and isolated muscle incubations. Am J Physiol. 1994 Jan;266(1 Pt 1):E1–16. doi: 10.1152/ajpendo.1994.266.1.E1. [DOI] [PubMed] [Google Scholar]
- Cartee G. D., Young D. A., Sleeper M. D., Zierath J., Wallberg-Henriksson H., Holloszy J. O. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989 Apr;256(4 Pt 1):E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Cawthorne M. A., Sennitt M. V., Arch J. R., Smith S. A. BRL 35135, a potent and selective atypical beta-adrenoceptor agonist. Am J Clin Nutr. 1992 Jan;55(1 Suppl):252S–257S. doi: 10.1093/ajcn/55.1.252s. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Leighton B., Wilson S., Thurlby P. L., Arch J. R. An investigation of the beta-adrenoceptor that mediates metabolic responses to the novel agonist BRL28410 in rat soleus muscle. Biochem Pharmacol. 1988 Mar 1;37(5):947–950. doi: 10.1016/0006-2952(88)90186-4. [DOI] [PubMed] [Google Scholar]
- Chaudhry A., MacKenzie R. G., Georgic L. M., Granneman J. G. Differential interaction of beta 1- and beta 3-adrenergic receptors with Gi in rat adipocytes. Cell Signal. 1994 May;6(4):457–465. doi: 10.1016/0898-6568(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., Smith R. J., Horton E. S. Glucose transporter number, activity, and isoform content in plasma membranes of red and white skeletal muscle. Am J Physiol. 1991 Nov;261(5 Pt 1):E556–E561. doi: 10.1152/ajpendo.1991.261.5.E556. [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol. 1991 Dec;40(6):895–899. [PubMed] [Google Scholar]
- Granneman J. G. Why do adipocytes make the beta 3 adrenergic receptor? Cell Signal. 1995 Jan;7(1):9–15. doi: 10.1016/0898-6568(94)00066-k. [DOI] [PubMed] [Google Scholar]
- Hollenga C., Brouwer F., Zaagsma J. Differences in functional cyclic AMP compartments mediating lipolysis by isoprenaline and BRL 37344 in four adipocyte types. Eur J Pharmacol. 1991 Aug 6;200(2-3):325–330. doi: 10.1016/0014-2999(91)90590-m. [DOI] [PubMed] [Google Scholar]
- Issad T., Pénicaud L., Ferré P., Kandé J., Baudon M. A., Girard J. Effects of fasting on tissue glucose utilization in conscious resting rats. Major glucose-sparing effect in working muscles. Biochem J. 1987 Aug 15;246(1):241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Klip A., Pâquet M. R. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990 Mar;13(3):228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- Krief S., Lönnqvist F., Raimbault S., Baude B., Van Spronsen A., Arner P., Strosberg A. D., Ricquier D., Emorine L. J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993 Jan;91(1):344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langin D., Ekholm D., Ridderstråle M., Lafontan M., Belfrage P. cAMP-dependent protein kinase activation mediated by beta 3-adrenergic receptors parallels lipolysis in rat adipocytes. Biochim Biophys Acta. 1992 Jun 29;1135(3):349–352. doi: 10.1016/0167-4889(92)90242-4. [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Stock M. J. Acute effects of the beta 3-adrenoceptor agonist, BRL 35135, on tissue glucose utilisation. Br J Pharmacol. 1995 Feb;114(4):888–894. doi: 10.1111/j.1476-5381.1995.tb13287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin D. P., MacDonald A. Evidence for the existence of 'atypical' beta-adrenoceptors (beta 3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br J Pharmacol. 1990 Nov;101(3):569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Molenaar P., Roberts S. J., Kim Y. S., Pak H. S., Sainz R. D., Summers R. J. Localization and characterization of two propranolol resistant (-) [125I]cyanopindolol binding sites in rat skeletal muscle. Eur J Pharmacol. 1991 Dec 17;209(3):257–262. doi: 10.1016/0014-2999(91)90179-t. [DOI] [PubMed] [Google Scholar]
- Murphy G. J., Kirkham D. M., Cawthorne M. A., Young P. Correlation of beta 3-adrenoceptor-induced activation of cyclic AMP-dependent protein kinase with activation of lipolysis in rat white adipocytes. Biochem Pharmacol. 1993 Aug 17;46(4):575–581. doi: 10.1016/0006-2952(93)90540-d. [DOI] [PubMed] [Google Scholar]
- Pessin J. E., Bell G. I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Molenaar P., Summers R. J. Characterization of propranolol-resistant (-)-[125I]-cyanopindolol binding sites in rat soleus muscle. Br J Pharmacol. 1993 Jun;109(2):344–352. doi: 10.1111/j.1476-5381.1993.tb13576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. T., Zhang H., Vatner S. F. Peripheral vascular effects of beta-3 adrenergic receptor stimulation in conscious dogs. J Pharmacol Exp Ther. 1994 Jan;268(1):466–473. [PubMed] [Google Scholar]
- Sillence M. N., Matthews M. L. Classical and atypical binding sites for beta-adrenoceptor ligands and activation of adenylyl cyclase in bovine skeletal muscle and adipose tissue membranes. Br J Pharmacol. 1994 Mar;111(3):866–872. doi: 10.1111/j.1476-5381.1994.tb14818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence M. N., Moore N. G., Pegg G. G., Lindsay D. B. Ligand binding properties of putative beta 3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br J Pharmacol. 1993 Aug;109(4):1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Sudo M., Minokoshi Y., Shimazu T. Effects of ventromedial hypothalamic stimulation on glucose transport system in rat tissues. Am J Physiol. 1992 Dec;263(6 Pt 2):R1228–R1234. doi: 10.1152/ajpregu.1992.263.6.R1228. [DOI] [PubMed] [Google Scholar]
- Thurlby P. L., Ellis R. D. Differences between the effects of noradrenaline and the beta-adrenoceptor agonist BRL 28410 in brown adipose tissue and hind limb of the anaesthetized rat. Can J Physiol Pharmacol. 1986 Aug;64(8):1111–1114. doi: 10.1139/y86-189. [DOI] [PubMed] [Google Scholar]
- Vallerand A. L., Pérusse F., Bukowiecki L. J. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol. 1987 Aug;253(2 Pt 1):E179–E186. doi: 10.1152/ajpendo.1987.253.2.E179. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol. 1985 Sep;249(3 Pt 1):C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- Yen T. T. Antiobesity and antidiabetic beta-agonists: lessons learned and questions to be answered. Obes Res. 1994 Sep;2(5):472–480. doi: 10.1002/j.1550-8528.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Sakane N., Wakabayashi Y., Umekawa T., Kondo M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994;54(7):491–498. doi: 10.1016/0024-3205(94)00408-0. [DOI] [PubMed] [Google Scholar]