Abstract
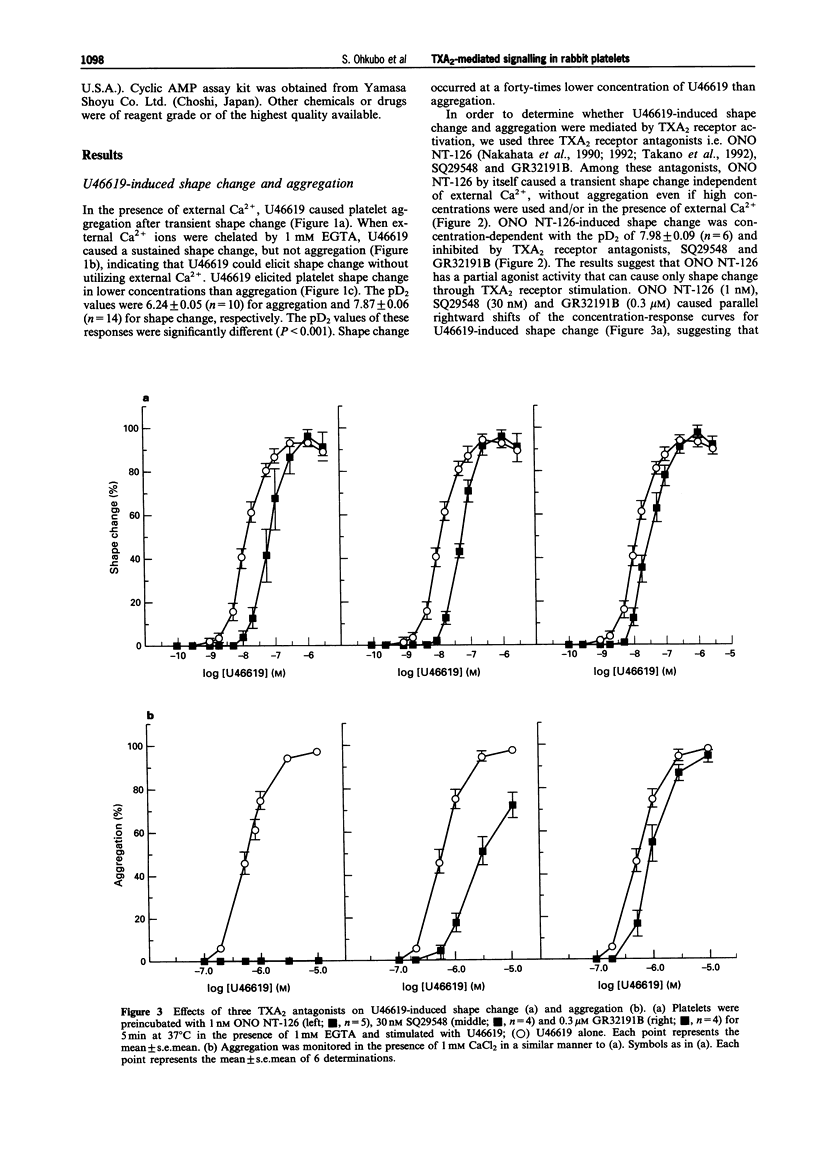
1. Thromboxane A2 (TXA2) receptor-mediated signal transduction was investigated in washed rabbit platelets to clarify the mechanisms of induction of shape change and aggregation. 2. The TXA2 agonist, U46619 (1 nM to 10 microM) caused shape change and aggregation in a concentration-dependent manner. A forty-times higher concentration of U46619 was needed for aggregation (EC50 of 0.58 microM) than shape change (EC50 of 0.013 microM). The aggregation occurred only when external 1 mM Ca2+ was present, but the shape change could occur in the absence of Ca2+. 3. SQ29548 at 30 nM and GR32191B at 0.3 microM (TXA2 receptor antagonists) competitively inhibited U46619-induced shape change and aggregation with similar potency, showing that both aggregation and shape change induced by U46619 were TXA2 receptor-mediated events. However, ONO NT-126 at 1 nM, another TXA2 receptor antagonist, inhibited U46619-induced aggregation much more potently than the shape change, suggesting the possible existence of TXA2 receptor subtypes. 4. ONO NT-126 (2 nM to 3 microM) by itself caused a shape change without aggregation in a concentration-dependent manner, independent of external Ca2+. Therefore, ONO NT-126 is a partial agonist at the TXA2 receptor in rabbit platelets. 5. U46619 (10 nM to 10 microM) increased internal Ca2+ concentration ([Ca2+]i) and activated phosphoinositide (PI) hydrolysis in a concentration-dependent manner with a similar concentration-dependency. 6. U46619 (3 nM to 10 microM) also activated GTPase concentration-dependently in the membranes derived from platelets. U46619-induced activation of GTPase was partly inhibited by treatment of membranes with QL, an antibody against Gq/11. 7. The EC50 values of U46619 in Ca2+ mobilization (0.15 microM), PI hydrolysis (0.20 microM) and increase in GTPase activity (0.12 microM) were similar, but different from the EC50 value in shape change (0.013 microM), suggesting that activation of TXA2 receptors might cause shape change via an unknown mechanism. 8. U46619-induced shape change was unaffected by W-7 (30 microM), a calmodulin antagonist or ML-7 (30 microM), a myosin light-chain kinase inhibitor, indicating that an increase in [Ca2+]i might not be involved in the shape change. In fact, U46619 (10 nM) could cause shape change without affecting [Ca2+]i level, determined by simultaneous recordings. 9. [3H]-SQ29548 and [3H]-U46619 bound to platelets at a single site with a Kd value of 14.88 nM and Bmax of 106.1 fmol/10(8) platelets and a Kd value of 129.8 nM and Bmax of 170.4 fmol/10(8) platelets, respectively. The inhibitory constant Ki value for U46619 as an inhibitor of 3H-ligand binding was similar to the EC50 value of U46619 in GTPase activity, phosphoinositide hydrolysis and Ca2+ mobilization, but significantly different (P < 0.001 by Student's t test) from the effect on shape change. 10. Neither U46619 nor ONO NT-126 affected the adenosine 3',5'-cyclic monophosphate (cyclic AMP) level in the presence or absence of external Ca2+ and/or isobutyl methylxanthine. 11. The results indicate that TXA2 receptor stimulation causes phospholipase C activation and increase in [Ca2+]i via a G protein of the Gq/11 family leading to aggregation in the presence of external Ca2+, and that shape change induced by TXA2 receptor stimulation might occur without involvement of the Gq-phospholipase C-Ca2+ pathway.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Armstrong R. A., Humphrey P. P., Lumley P. Characteristics of the binding of [3H]-GR32191 to the thromboxane (TP-) receptor of human platelets. Br J Pharmacol. 1993 Oct;110(2):539–547. doi: 10.1111/j.1476-5381.1993.tb13844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A., Humphrey P. P., Lumley P. Characteristics of the binding of [3H]-GR32191 to the thromboxane (TP-) receptor of human platelets. Br J Pharmacol. 1993 Oct;110(2):539–547. doi: 10.1111/j.1476-5381.1993.tb13844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassare J. J., Tarver A. P., Henderson P. A., Mackin W. M., Sahagan B., Fisher G. J. Reconstitution of thromboxane A2 receptor-stimulated phosphoinositide hydrolysis in isolated platelet membranes: involvement of phosphoinositide-specific phospholipase C-beta and GTP-binding protein Gq. Biochem J. 1993 Apr 1;291(Pt 1):235–240. doi: 10.1042/bj2910235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Borg C., Lim C. T., Yeomans D. C., Dieter J. P., Komiotis D., Anderson E. G., Le Breton G. C. Purification of rat brain, rabbit aorta, and human platelet thromboxane A2/prostaglandin H2 receptors by immunoaffinity chromatography employing anti-peptide and anti-receptor antibodies. J Biol Chem. 1994 Feb 25;269(8):6109–6116. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Shaller C. C., Belmonte E. J. Inositol 1,4,5-triphosphate-induced granule secretion in platelets. Evidence that the activation of phospholipase C mediated by platelet thromboxane receptors involves a guanine nucleotide binding protein-dependent mechanism distinct from that of thrombin. J Clin Invest. 1987 Apr;79(4):1269–1275. doi: 10.1172/JCI112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dorn G. W., 2nd, DeJesus A. Human platelet aggregation and shape change are coupled to separate thromboxane A2-prostaglandin H2 receptors. Am J Physiol. 1991 Feb;260(2 Pt 2):H327–H334. doi: 10.1152/ajpheart.1991.260.2.H327. [DOI] [PubMed] [Google Scholar]
- Dorn G. W., 2nd Tissue- and species-specific differences in ligand binding to thromboxane A2 receptors. Am J Physiol. 1991 Jul;261(1 Pt 2):R145–R153. doi: 10.1152/ajpregu.1991.261.1.R145. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., White J. G. Ultrastructural features on the platelet response to phorbol myristate acetate. Am J Pathol. 1974 Mar;74(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Fujimura K., Kuramoto A. Electrophysiological evidence that glycoprotein IIb-IIIa complex is involved in calcium channel activation on human platelet plasma membrane. J Biol Chem. 1991 Sep 5;266(25):16370–16375. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Furci L., Fitzgerald D. J., Fitzgerald G. A. Heterogeneity of prostaglandin H2/thromboxane A2 receptors: distinct subtypes mediate vascular smooth muscle contraction and platelet aggregation. J Pharmacol Exp Ther. 1991 Jul 1;258(1):74–81. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gusovsky F., Lueders J. E., Kohn E. C., Felder C. C. Muscarinic receptor-mediated tyrosine phosphorylation of phospholipase C-gamma. An alternative mechanism for cholinergic-induced phosphoinositide breakdown. J Biol Chem. 1993 Apr 15;268(11):7768–7772. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Honma M., Satoh T., Takezawa J., Ui M. An ultrasensitive method for the simultaneous determination of cyclic AMP and cyclic GMP in small-volume samples from blood and tissue. Biochem Med. 1977 Dec;18(3):257–273. doi: 10.1016/0006-2944(77)90060-6. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986 Mar 15;234(3):737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Hara T., Yamada F., Shibata N. Diphosphorylation of platelet myosin ex vivo in the initial phase of activation by thrombin. Biochim Biophys Acta. 1992 Jul 22;1136(1):52–56. doi: 10.1016/0167-4889(92)90084-o. [DOI] [PubMed] [Google Scholar]
- Knezevic I., Borg C., Le Breton G. C. Identification of Gq as one of the G-proteins which copurify with human platelet thromboxane A2/prostaglandin H2 receptors. J Biol Chem. 1993 Dec 5;268(34):26011–26017. [PubMed] [Google Scholar]
- Kucera G. L., Rittenhouse S. E. Human platelets form 3-phosphorylated phosphoinositides in response to alpha-thrombin, U46619, or GTP gamma S. J Biol Chem. 1990 Apr 5;265(10):5345–5348. [PubMed] [Google Scholar]
- Lefer A. M., Smith E. F., 3rd, Araki H., Smith J. B., Aharony D., Claremon D. A., Magolda R. L., Nicolaou K. C. Dissociation of vasoconstrictor and platelet aggregatory activities of thromboxane by carbocyclic thromboxane A2, a stable analog of thromboxane A2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1706–1710. doi: 10.1073/pnas.77.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley P., White B. P., Humphrey P. P. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br J Pharmacol. 1989 Jul;97(3):783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Taniguchi T., Inazu T., Yang C., Nakagawara G., Yamamura H. Protein-tyrosine kinase p72syk is activated by thromboxane A2 mimetic U44069 in platelets. Biochem Biophys Res Commun. 1993 Nov 30;197(1):62–67. doi: 10.1006/bbrc.1993.2441. [DOI] [PubMed] [Google Scholar]
- Mais D. E., Saussy D. L., Jr, Chaikhouni A., Kochel P. J., Knapp D. R., Hamanaka N., Halushka P. V. Pharmacologic characterization of human and canine thromboxane A2/prostaglandin H2 receptors in platelets and blood vessels: evidence for different receptors. J Pharmacol Exp Ther. 1985 May;233(2):418–424. [PubMed] [Google Scholar]
- Mayeux P. R., Morinelli T. A., Williams T. C., Hazard E. S., Mais D. E., Oatis J. E., Baron D. A., Halushka P. V. Differential effect of pH on thromboxane A2/prostaglandin H2 receptor agonist and antagonist binding in human platelets. J Biol Chem. 1991 Jul 25;266(21):13752–13758. [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinelli T. A., Zhang L. M., Newman W. H., Meier K. E. Thromboxane A2/prostaglandin H2-stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J Biol Chem. 1994 Feb 25;269(8):5693–5698. [PubMed] [Google Scholar]
- Murray R., Shipp E., FitzGerald G. A. Prostaglandin endoperoxide/thromboxane A2 receptor desensitization. Cross-talk with adenylate cyclase in human platelets. J Biol Chem. 1990 Dec 15;265(35):21670–21675. [PubMed] [Google Scholar]
- Nakahata N., Ishimoto H., Kurita M., Ohmori K., Takahashi A., Nakanishi H. The presence of thromboxane A2 receptors in cultured astrocytes from rabbit brain. Brain Res. 1992 Jun 26;583(1-2):100–104. doi: 10.1016/s0006-8993(10)80013-7. [DOI] [PubMed] [Google Scholar]
- Nakahata N., Ishimoto H., Mizuno K., Ohizumi Y., Nakanishi H. Dual effects of mastoparan on intracellular free Ca2+ concentrations in human astrocytoma cells. Br J Pharmacol. 1994 May;112(1):299–303. doi: 10.1111/j.1476-5381.1994.tb13068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata N., Sato K., Abe M. T., Nakanishi H. ONO NT-126 is a potent and selective thromboxane A2 antagonist in human astrocytoma cells. Eur J Pharmacol. 1990 Aug 10;184(2-3):233–238. doi: 10.1016/0014-2999(90)90614-c. [DOI] [PubMed] [Google Scholar]
- Narumiya S., Okuma M., Ushikubi F. Binding of a radioiodinated 13-azapinane thromboxane antagonist to platelets: correlation with antiaggregatory activity in different species. Br J Pharmacol. 1986 Jun;88(2):323–331. doi: 10.1111/j.1476-5381.1986.tb10208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Oda A., Druker B. J., Smith M., Salzman E. W. Association of pp60src with Triton X-100-insoluble residue in human blood platelets requires platelet aggregation and actin polymerization. J Biol Chem. 1992 Oct 5;267(28):20075–20081. [PubMed] [Google Scholar]
- Offermanns S., Laugwitz K. L., Spicher K., Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Raychowdhury M. K., Yukawa M., Collins L. J., McGrail S. H., Kent K. C., Ware J. A. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J Biol Chem. 1994 Jul 29;269(30):19256–19261. [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Siess W., Boehlig B., Weber P. C., Lapetina E. G. Prostaglandin endoperoxide analogues stimulate phospholipase C and protein phosphorylation during platelet shape change. Blood. 1985 May;65(5):1141–1148. [PubMed] [Google Scholar]
- Simpson A. W., Hallam T. J., Rink T. J. Low concentrations of the stable prostaglandin endoperoxide U44069 stimulate shape change in quin2-loaded platelets without a measurable increase in [Ca2+]i. FEBS Lett. 1986 Jun 9;201(2):301–305. doi: 10.1016/0014-5793(86)80628-7. [DOI] [PubMed] [Google Scholar]
- Takahara K., Murray R., FitzGerald G. A., Fitzgerald D. J. The response to thromboxane A2 analogues in human platelets. Discrimination of two binding sites linked to distinct effector systems. J Biol Chem. 1990 Apr 25;265(12):6836–6844. [PubMed] [Google Scholar]
- Takano S., Ishimoto H., Nakahata N., Nakanishi H. Thromboxane A2 receptor characterization in human astrocytoma cells and rabbit platelets by a new thromboxane antagonist, [3H]ONO NT-126. Res Commun Chem Pathol Pharmacol. 1992 May;76(2):155–170. [PubMed] [Google Scholar]
- Ushikubi F., Nakamura K., Narumiya S. Functional reconstitution of platelet thromboxane A2 receptors with Gq and Gi2 in phospholipid vesicles. Mol Pharmacol. 1994 Nov;46(5):808–816. [PubMed] [Google Scholar]


