Abstract
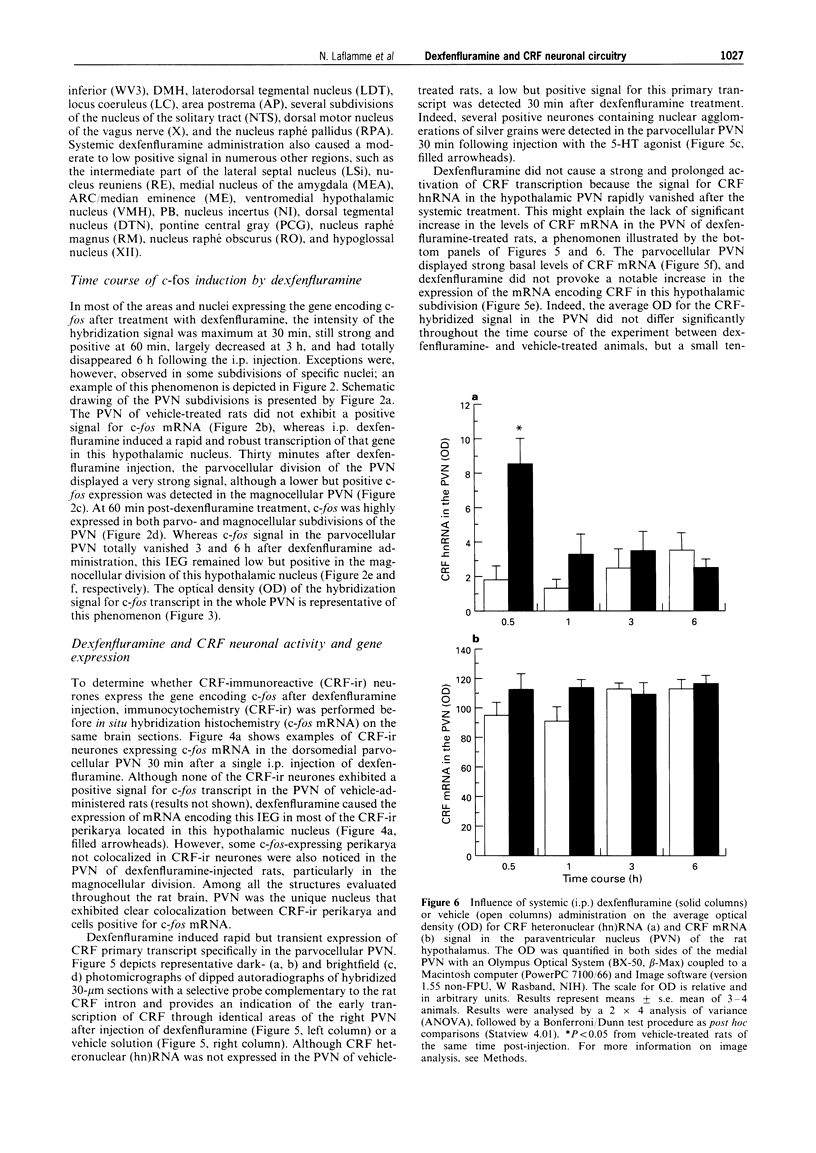
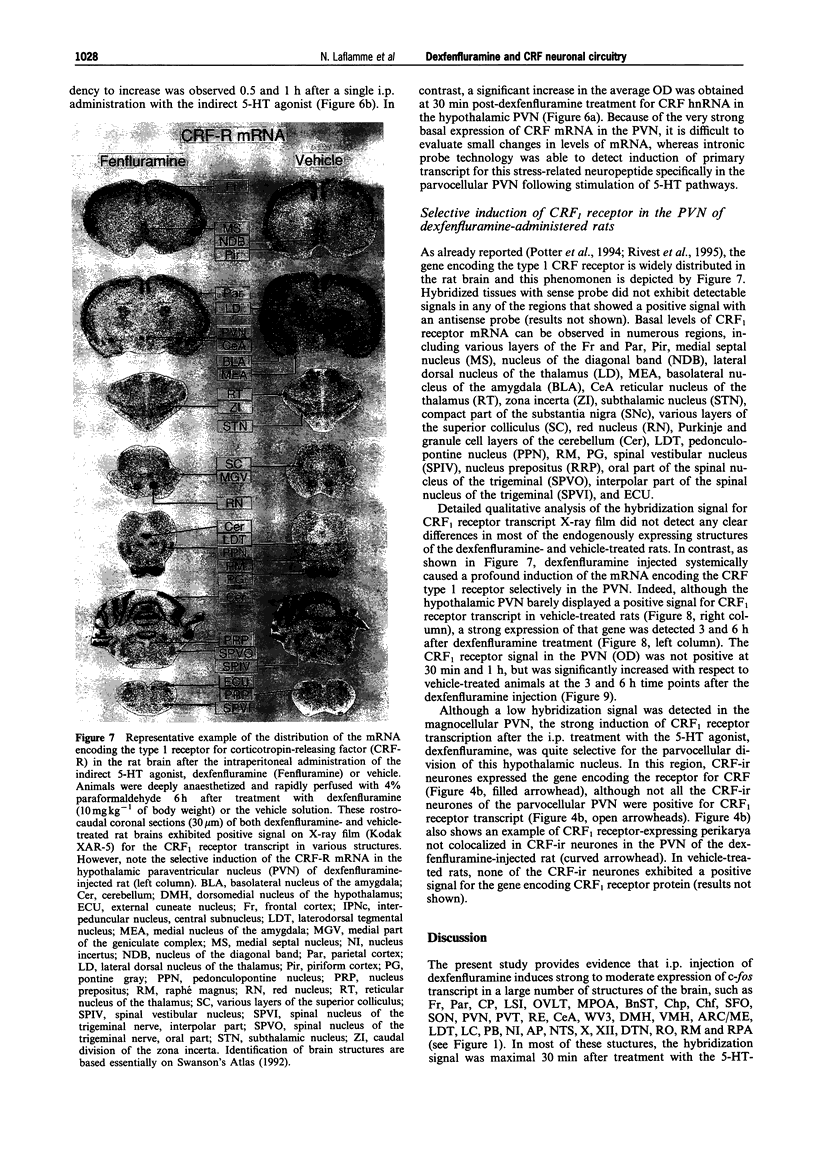
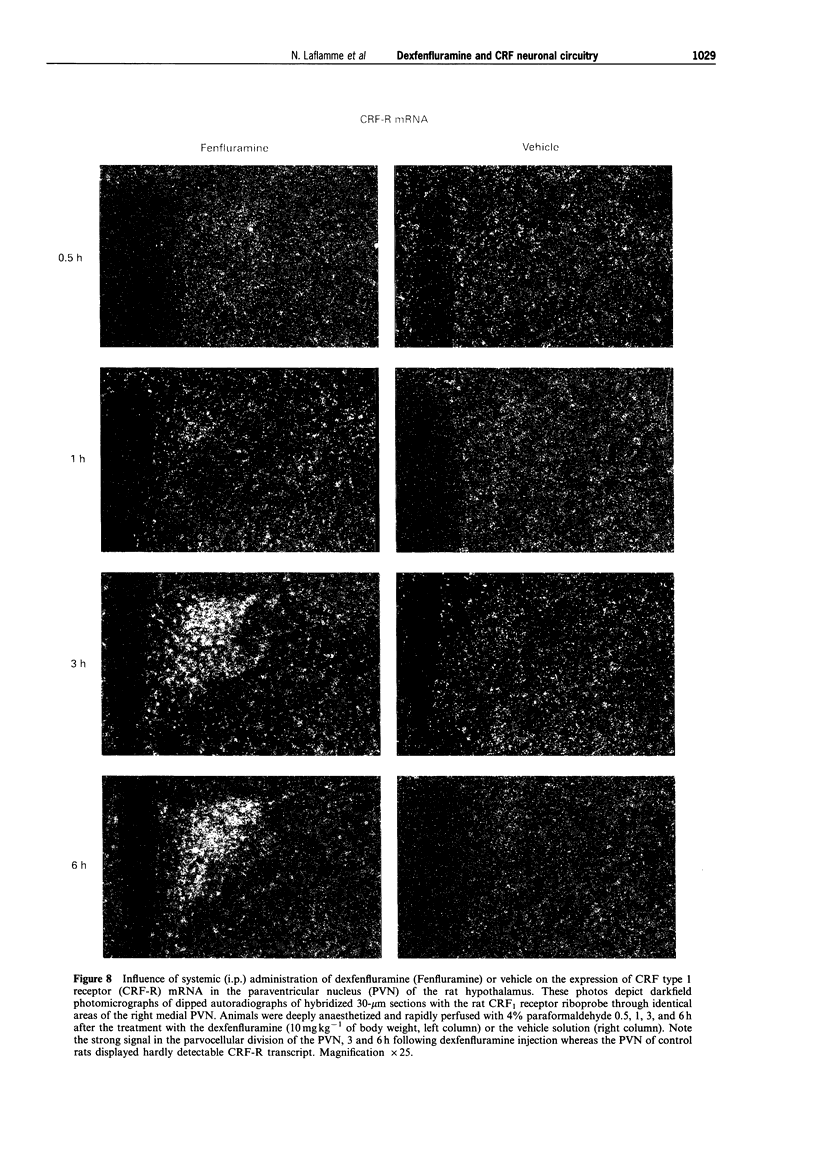
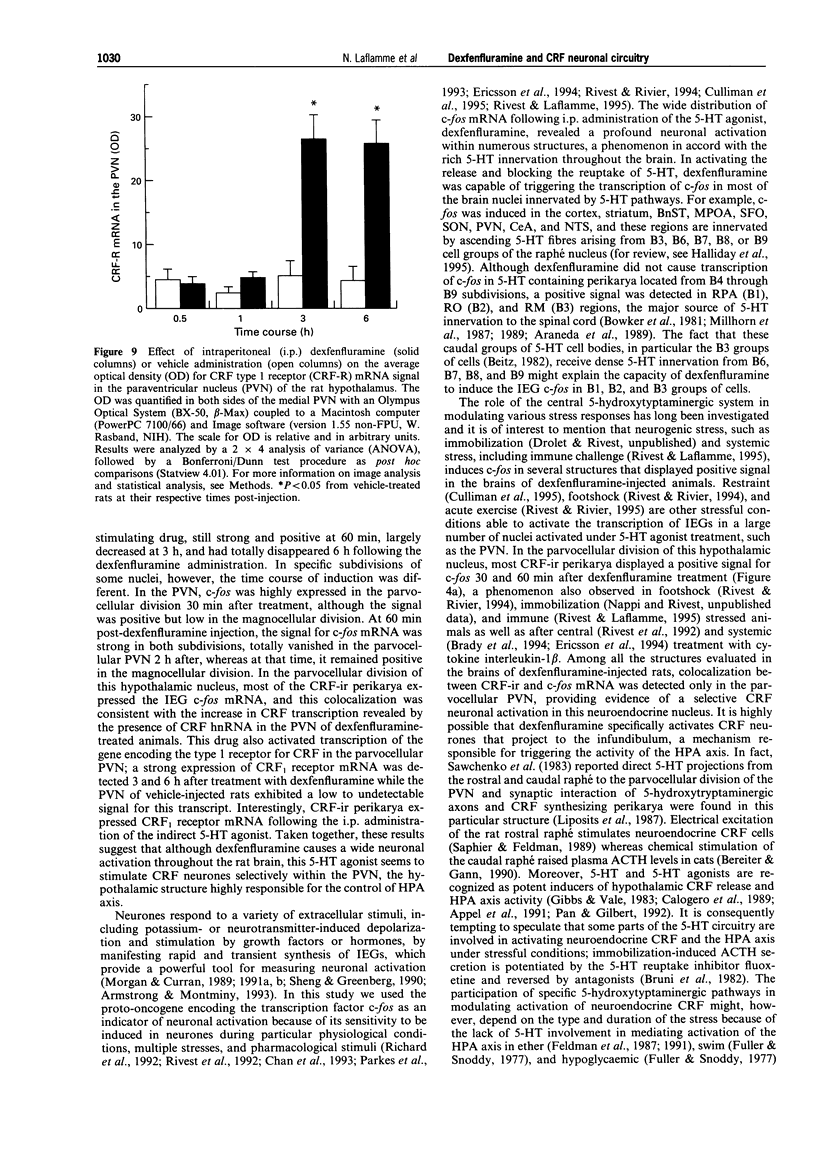
1. The present study investigated the effect of intraperitoneal (i.p.) administration of the indirect 5-hydroxytryptamine (5-HT) receptor agonist, dexfenfluramine, on the transcriptional activity of corticotropin-releasing factor (CRF) and its type 1 receptor in the brains of conscious male Sprague-Dawley rats via in situ hybridization histochemistry (ISHH) using both intronic and exonic probe technology. 2. The immediate early gene (IEG) c-fos mRNA was also used as index of cellular activity, whereas localization between CRF-immunoreactive (ir) perikarya and the IEG was accomplished to determine the site of CRF neuronal activation in the brain of dexfenfluramine-treated rats. 3. Thirty minutes, 1, 3, and 6 h after a single injection of either dexfenfluramine (10 mg kg-1) or the vehicle solution, adult male rats (230-260 g) were deeply anaesthetized and rapidly perfused with a 4% paraformaldehyde-borax solution (PF). The brains were removed from the skull, postfixed, and placed in a solution of 4% PF-10% sucrose overnight at 4 degrees C. Frozen brains were mounted on a microtome and cut from the olfactory bulb to the medulla in 30-microns coronal sections. 4. Dexfenfluramine induced a general neuronal activation as indicated by the strong signal of c-fos mRNA in several structures of the brain, including the parietal cortex, caudate putamen, circumventricular organs, medial preoptic area, bed nucleus of the stria terminalis, choroid plexus, choroidal fissure, supraoptic nucleus, paraventricular nucleus of the hypothalamus (PVN), paraventricular nucleus of the thalamus, central nucleus of the amygdala, dorsomedial nucleus of the hypothalamus, laterodorsal tegmental nucleus, locus coeruleus, and several subdivisions of the dorsal vagal complex. In most of these structures, the signal was maximal at 30 min, still strong and positive at 60 min, largely decreased at 3 h, and had completely disappeared 6 h after injection. 5. In the parvocellular division of the PVN, the large majority of CRF-ir perikarya displayed a positive signal for the mRNA encoding c-fos, indicating a profound CRFergic activation within this neuroendocrine nucleus after dexfenfluramine administration. 6. Colocalization between CRF-ir neurones and c-fos positive cells was not detected in any other regions. This selective activation of PVN CRF neurones was also confirmed by the presence of CRF primary transcript; 30 min after i.p. injection of the indirect 5-HT agonist, a positive signal for CRF hnRNA was observed, specifically in the parvocellular PVN. 7. Transcription of the gene encoding the type 1 receptor for CRF was highly stimulated in the PVN following 5-HT activation. Although this hypothalamic nucleus exhibited a barely detectable signal under basal conditions, dexfenfluramine induced a strong signal of CRF1 receptor mRNA in the parvocellular PVN. Interestingly, CRF-ir neurones displayed a positive signal for the mRNA encoding the CRF1 receptor, 3 and 6 h after systemic treatment with dexfenfluramine. 8. These results indicate that although dexfenfluramine can generate a wide neuronal activation throughout the brain, this 5-HT agonist triggers the activity of CRF neurones selectively in the parvocellular division of the PVN, a mechanism possibly related to the activity of hypothalamic-pituitary-adrenal axis. Induction of CRF1 receptor mRNA in CRF cells of the PVN indicates that neuroendocrine CRF neurones can be targeted by CNS CRF under 5-HT stimulation.
Full text
PDF

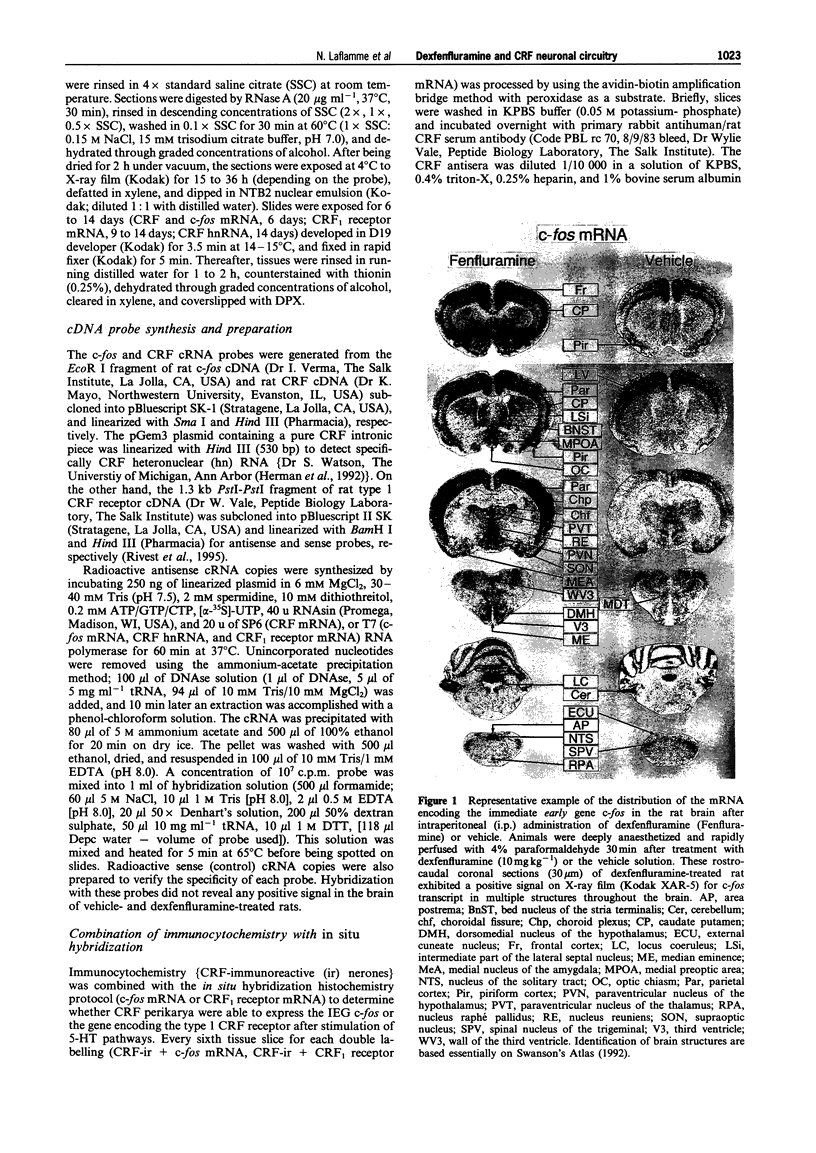




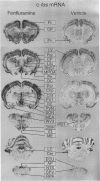
Images in this article
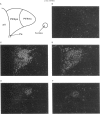
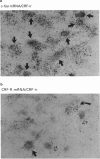
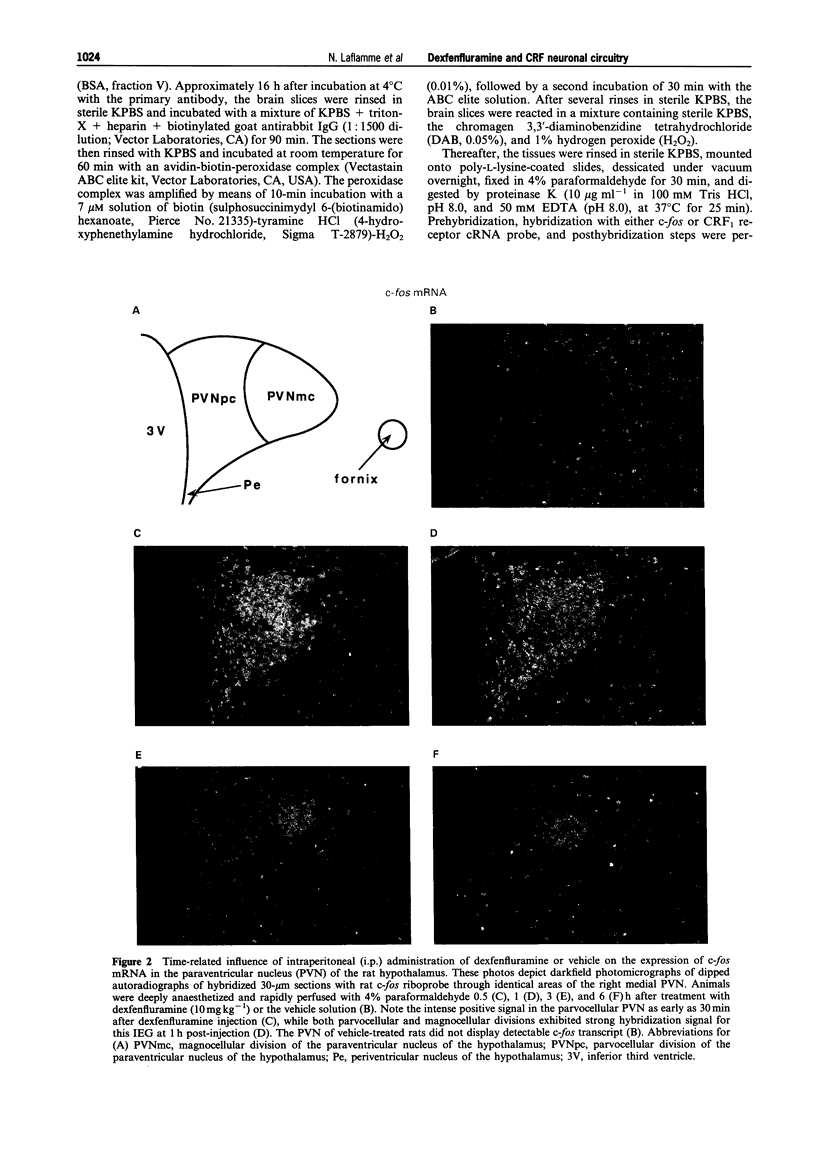
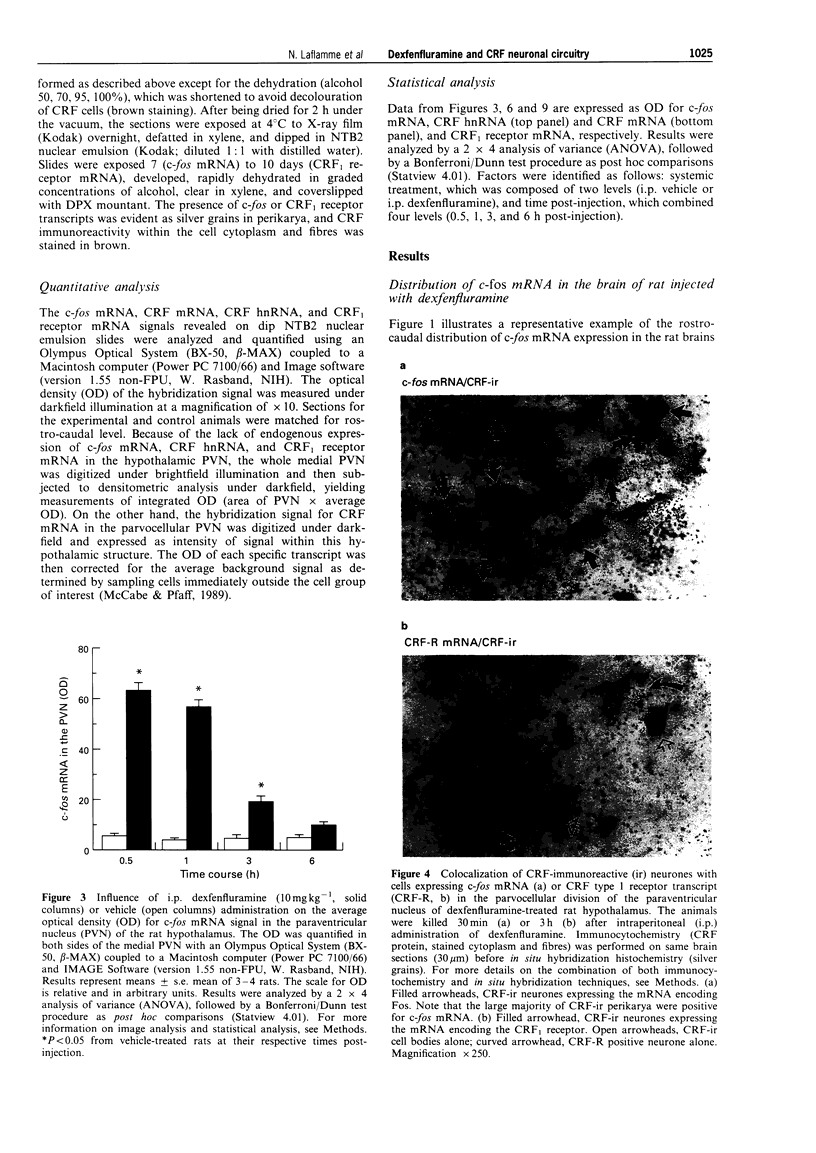
Selected References
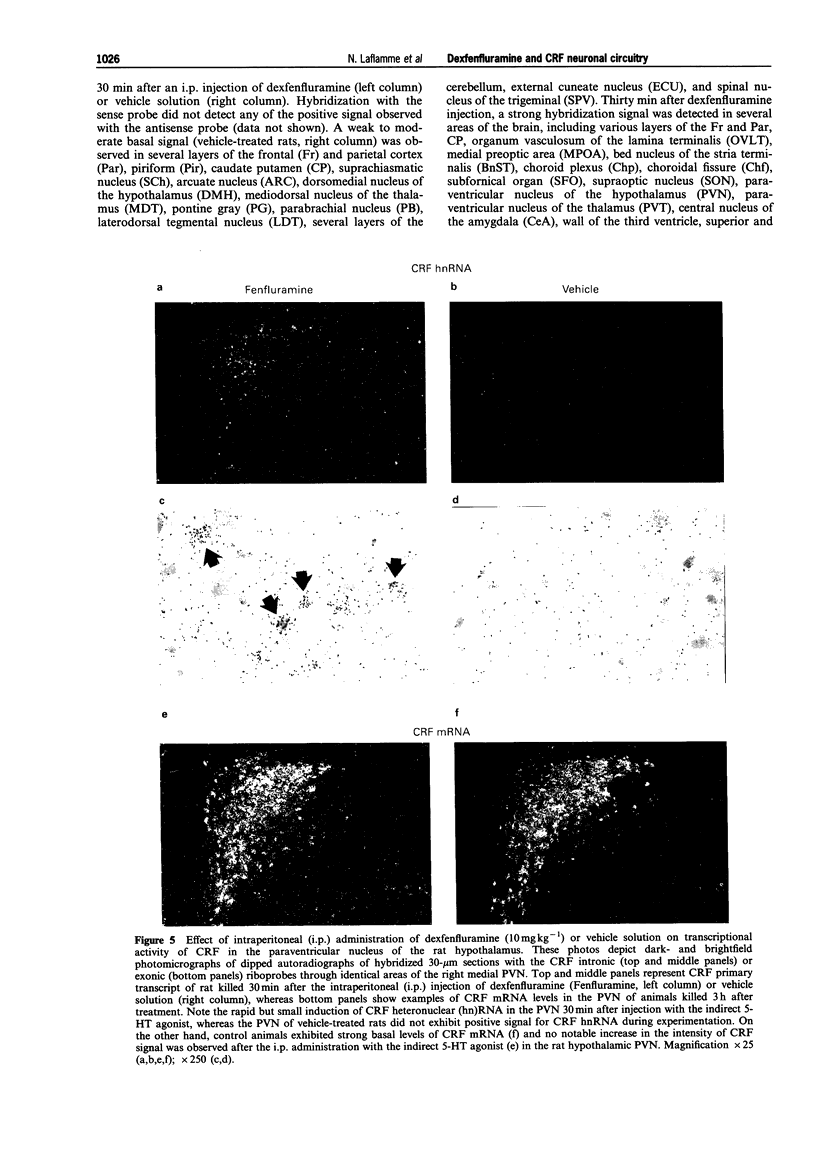
These references are in PubMed. This may not be the complete list of references from this article.
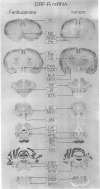
- Alper R. H. Evidence for central and peripheral serotonergic control of corticosterone secretion in the conscious rat. Neuroendocrinology. 1990 Mar;51(3):255–260. doi: 10.1159/000125347. [DOI] [PubMed] [Google Scholar]
- Appel N. M., Owens M. J., Culp S., Zaczek R., Contrera J. F., Bissette G., Nemeroff C. B., De Souza E. B. Role for brain corticotropin-releasing factor in the weight-reducing effects of chronic fenfluramine treatment in rats. Endocrinology. 1991 Jun;128(6):3237–3246. doi: 10.1210/endo-128-6-3237. [DOI] [PubMed] [Google Scholar]
- Araneda S., Magoul R., Calas A. Tracing specific transmitter pathways in the rat CNS: combination of [3H]serotonin retrograde labelling with immunocytochemical detection of endogenous transmitters. J Neurosci Methods. 1989 Dec;30(3):211–218. doi: 10.1016/0165-0270(89)90132-5. [DOI] [PubMed] [Google Scholar]
- Armstrong R. C., Montminy M. R. Transsynaptic control of gene expression. Annu Rev Neurosci. 1993;16:17–29. doi: 10.1146/annurev.ne.16.030193.000313. [DOI] [PubMed] [Google Scholar]
- Assenmacher I., Szafarczyk A., Alonso G., Ixart G., Barbanel G. Physiology of neural pathways affecting CRH secretion. Ann N Y Acad Sci. 1987;512:149–161. doi: 10.1111/j.1749-6632.1987.tb24957.x. [DOI] [PubMed] [Google Scholar]
- Bagdy G., Calogero A. E., Murphy D. L., Szemeredi K. Serotonin agonists cause parallel activation of the sympathoadrenomedullary system and the hypothalamo-pituitary-adrenocortical axis in conscious rats. Endocrinology. 1989 Nov;125(5):2664–2669. doi: 10.1210/endo-125-5-2664. [DOI] [PubMed] [Google Scholar]
- Beitz A. J. The sites of origin brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci. 1982 Jul;2(7):829–842. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter D. A., Gann D. S. Comparison of the influence of rostral and caudal raphe neurons on the adrenal secretion of catecholamines and on the release of adrenocorticotropin in the cat. Pain. 1990 Jul;42(1):81–91. doi: 10.1016/0304-3959(90)91094-Y. [DOI] [PubMed] [Google Scholar]
- Bowker R. M., Westlund K. N., Coulter J. D. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res. 1981 Dec 7;226(1-2):187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Brady L. S., Lynn A. B., Herkenham M., Gottesfeld Z. Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci. 1994 Aug;14(8):4951–4964. doi: 10.1523/JNEUROSCI.14-08-04951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A., Spiess J., Rivier C., Rivier J., Vale W. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology. 1982 Sep;111(3):928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A., Webb V., Vale W. W., Rivier J. E. Corticotropin-releasing factor: a physiologic regulator of adrenal epinephrine secretion. Brain Res. 1985 Mar 4;328(2):355–357. doi: 10.1016/0006-8993(85)91048-0. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Gray T. S., Fisher L. A. Corticotropin-releasing factor receptor antagonist: effects on the autonomic nervous system and cardiovascular function. Regul Pept. 1986 Dec 30;16(3-4):321–329. doi: 10.1016/0167-0115(86)90032-7. [DOI] [PubMed] [Google Scholar]
- Bruni J. F., Hawkins R. L., Yen S. S. Serotonergic mechanism in the control of beta-endorphin and ACTH release in male rats. Life Sci. 1982 Apr 12;30(15):1247–1254. doi: 10.1016/0024-3205(82)90686-5. [DOI] [PubMed] [Google Scholar]
- Calogero A. E., Bernardini R., Margioris A. N., Bagdy G., Gallucci W. T., Munson P. J., Tamarkin L., Tomai T. P., Brady L., Gold P. W. Effects of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989 Jan-Feb;10(1):189–200. doi: 10.1016/0196-9781(89)90096-x. [DOI] [PubMed] [Google Scholar]
- Calogero A. E., Sternberg E. M., Bagdy G., Smith C., Bernardini R., Aksentijevich S., Wilder R. L., Gold P. W., Chrousos G. P. Neurotransmitter-induced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible Lewis rats: in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone. Neuroendocrinology. 1992 May;55(5):600–608. doi: 10.1159/000126173. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Brown E. R., Ericsson A., Kovács K. J., Sawchenko P. E. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993 Dec;13(12):5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W. E., Herman J. P., Battaglia D. F., Akil H., Watson S. J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995 Jan;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dunn A. J., Berridge C. W. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990 May-Aug;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Kovács K. J., Sawchenko P. E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994 Feb;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S., Conforti N., Melamed E. Paraventricular nucleus serotonin mediates neurally stimulated adrenocortical secretion. Brain Res Bull. 1987 Feb;18(2):165–168. doi: 10.1016/0361-9230(87)90186-9. [DOI] [PubMed] [Google Scholar]
- Feldman S., Weidenfeld J., Conforti N., Saphier D. Differential recovery of adrenocortical responses to neural stimuli following administration of 5,7-dihydroxytryptamine into the hypothalamus. Exp Brain Res. 1991;85(1):144–148. doi: 10.1007/BF00229995. [DOI] [PubMed] [Google Scholar]
- Fisher L. A., Rivier J., Rivier C., Spiess J., Vale W., Brown M. R. Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology. 1982 Jun;110(6):2222–2224. doi: 10.1210/endo-110-6-2222. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Snoddy H. D. Elevation of plasma corticosterone by swim stress and insulin-induced hypoglycemia in control and fluoxetine-pretreated rats. Endocr Res Commun. 1977;4(1):11–23. doi: 10.1080/07435807709045730. [DOI] [PubMed] [Google Scholar]
- Gibbs D. M., Vale W. Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res. 1983 Nov 28;280(1):176–179. doi: 10.1016/0006-8993(83)91189-7. [DOI] [PubMed] [Google Scholar]
- Harbuz M. S., Chalmers J., De Souza L., Lightman S. L. Stress-induced activation of CRF and c-fos mRNAs in the paraventricular nucleus are not affected by serotonin depletion. Brain Res. 1993 Apr 23;609(1-2):167–173. doi: 10.1016/0006-8993(93)90870-s. [DOI] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Herman J. P., Schafer M. K., Thompson R. C., Watson S. J. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992 Jul;6(7):1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Holmes M. C., Di Renzo G., Beckford U., Gillham B., Jones M. T. Role of serotonin in the control of secretion of corticotrophin releasing factor. J Endocrinol. 1982 May;93(2):151–160. doi: 10.1677/joe.0.0930151. [DOI] [PubMed] [Google Scholar]
- Imaki T., Nahan J. L., Rivier C., Sawchenko P. E., Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991 Mar;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T., Shibasaki T., Hotta M., Demura H. Early induction of c-fos precedes increased expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilization stress. Endocrinology. 1992 Jul;131(1):240–246. doi: 10.1210/endo.131.1.1612001. [DOI] [PubMed] [Google Scholar]
- Imaki T., Vale W., Sawchenko P. E. Regulation of corticotropin-releasing factor mRNA in neuroendocrine and autonomic neurons by osmotic stimulation and volume loading. Neuroendocrinology. 1992 Nov;56(5):633–640. doi: 10.1159/000126286. [DOI] [PubMed] [Google Scholar]
- Kakucska I., Qi Y., Clark B. D., Lechan R. M. Endotoxin-induced corticotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is mediated centrally by interleukin-1. Endocrinology. 1993 Aug;133(2):815–821. doi: 10.1210/endo.133.2.8344218. [DOI] [PubMed] [Google Scholar]
- Le Feuvre R. A., Aisenthal L., Rothwell N. J. Involvement of corticotrophin releasing factor (CRF) in the thermogenic and anorexic actions of serotonin (5-HT) and related compounds. Brain Res. 1991 Aug 2;555(2):245–250. doi: 10.1016/0006-8993(91)90348-y. [DOI] [PubMed] [Google Scholar]
- Levine A. S., Rogers B., Kneip J., Grace M., Morley J. E. Effect of centrally administered corticotropin releasing factor (CRF) on multiple feeding paradigms. Neuropharmacology. 1983 Mar;22(3):337–339. doi: 10.1016/0028-3908(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Phelix C., Paull W. K. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86(6):541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Lovenberg T. W., Liaw C. W., Grigoriadis D. E., Clevenger W., Chalmers D. T., De Souza E. B., Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Kiss A., Makara G., Lolait S. J., Aguilera G. Stress-specific regulation of corticotropin releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Neuroendocrinol. 1994 Dec;6(6):689–696. doi: 10.1111/j.1365-2826.1994.tb00636.x. [DOI] [PubMed] [Google Scholar]
- McElroy J. F., Miller J. M., Meyer J. S. Fenfluramine, p-chloroamphetamine and p-fluoroamphetamine stimulation of pituitary-adrenocortical activity in rat: evidence for differences in site and mechanism of action. J Pharmacol Exp Ther. 1984 Mar;228(3):593–599. [PubMed] [Google Scholar]
- Millhorn D. E., Hökfelt T., Seroogy K., Oertel W., Verhofstad A. A., Wu J. Y. Immunohistochemical evidence for colocalization of gamma-aminobutyric acid and serotonin in neurons of the ventral medulla oblongata projecting to the spinal cord. Brain Res. 1987 Apr 28;410(1):179–185. doi: 10.1016/s0006-8993(87)80043-4. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Hökfelt T., Verhofstad A. A., Terenius L. Individual cells in the raphe nuclei of the medulla oblongata in rat that contain immunoreactivities for both serotonin and enkephalin project to the spinal cord. Exp Brain Res. 1989;75(3):536–542. doi: 10.1007/BF00249904. [DOI] [PubMed] [Google Scholar]
- Moga M. M., Saper C. B. Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol. 1994 Aug 1;346(1):137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Proto-oncogene transcription factors and epilepsy. Trends Pharmacol Sci. 1991 Sep;12(9):343–349. doi: 10.1016/0165-6147(91)90594-i. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nakagami Y., Suda T., Yajima F., Ushiyama T., Tomori N., Sumitomo T., Demura H., Shizume K. Effects of serotonin, cyproheptadine and reserpine on corticotropin-releasing factor release from the rat hypothalamus in vitro. Brain Res. 1986 Oct 29;386(1-2):232–236. doi: 10.1016/0006-8993(86)90159-9. [DOI] [PubMed] [Google Scholar]
- Nappi R. E., Rivest S. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology. 1995 Sep;136(9):4073–4083. doi: 10.1210/endo.136.9.7649116. [DOI] [PubMed] [Google Scholar]
- Oliver C., Jezová D., Grino M., Guillaume V., Boudouresque F., Conte-Devolx B., Pesce G., Dutour A., Becquet D. Differences in the effects of acute and chronic administration of dexfenfluramine on cortisol and prolactin secretion. Adv Exp Med Biol. 1990;274:427–443. doi: 10.1007/978-1-4684-5799-5_27. [DOI] [PubMed] [Google Scholar]
- Ono N., Bedran de Castro J. C., McCann S. M. Ultrashort-loop positive feedback of corticotropin (ACTH)-releasing factor to enhance ACTH release in stress. Proc Natl Acad Sci U S A. 1985 May;82(10):3528–3531. doi: 10.1073/pnas.82.10.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M. J., Edwards E., Nemeroff C. B. Effects of 5-HT1A receptor agonists on hypothalamo-pituitary-adrenal axis activity and corticotropin-releasing factor containing neurons in the rat brain. Eur J Pharmacol. 1990 Nov 6;190(1-2):113–122. doi: 10.1016/0014-2999(90)94118-h. [DOI] [PubMed] [Google Scholar]
- Owens M. J., Knight D. L., Ritchie J. C., Nemeroff C. B. The 5-hydroxytryptamine2 agonist, (+-)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane stimulates the hypothalamic-pituitary-adrenal (HPA) axis. I. Acute effects on HPA axis activity and corticotropin-releasing factor-containing neurons in the rat brain. J Pharmacol Exp Ther. 1991 Feb;256(2):787–794. [PubMed] [Google Scholar]
- Pan L., Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology. 1992 Dec;56(6):797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- Parkes D., Rivest S., Lee S., Rivier C., Vale W. Corticotropin-releasing factor activates c-fos, NGFI-B, and corticotropin-releasing factor gene expression within the paraventricular nucleus of the rat hypothalamus. Mol Endocrinol. 1993 Oct;7(10):1357–1367. doi: 10.1210/mend.7.10.8264665. [DOI] [PubMed] [Google Scholar]
- Perrin M., Donaldson C., Chen R., Blount A., Berggren T., Bilezikjian L., Sawchenko P., Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E., Sutton S., Donaldson C., Chen R., Perrin M., Lewis K., Sawchenko P. E., Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D., Rivest S., Rivier C. The 5-hydroxytryptamine agonist fenfluramine increases Fos-like immunoreactivity in the brain. Brain Res. 1992 Oct 23;594(1):131–137. doi: 10.1016/0006-8993(92)91037-f. [DOI] [PubMed] [Google Scholar]
- Rivest S., Deshaies Y., Richard D. Effects of corticotropin-releasing factor on energy balance in rats are sex dependent. Am J Physiol. 1989 Dec;257(6 Pt 2):R1417–R1422. doi: 10.1152/ajpregu.1989.257.6.R1417. [DOI] [PubMed] [Google Scholar]
- Rivest S., Laflamme N., Nappi R. E. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci. 1995 Apr;15(4):2680–2695. doi: 10.1523/JNEUROSCI.15-04-02680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S., Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol. 1995 Jul;7(7):501–525. doi: 10.1111/j.1365-2826.1995.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Rivest S., Rivier C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994 Feb;6(1):101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Rivest S., Rivier C. The role of corticotropin-releasing factor and interleukin-1 in the regulation of neurons controlling reproductive functions. Endocr Rev. 1995 Apr;16(2):177–199. doi: 10.1210/edrv-16-2-177. [DOI] [PubMed] [Google Scholar]
- Rivest S., Torres G., Rivier C. Differential effects of central and peripheral injection of interleukin-1 beta on brain c-fos expression and neuroendocrine functions. Brain Res. 1992 Jul 31;587(1):13–23. doi: 10.1016/0006-8993(92)91424-d. [DOI] [PubMed] [Google Scholar]
- Sakanaka M., Shibasaki T., Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987 Jun 8;260(2):256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Samanin R., Garattini S. Neurochemical mechanism of action of anorectic drugs. Pharmacol Toxicol. 1993 Aug;73(2):63–68. doi: 10.1111/j.1600-0773.1993.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Saphier D., Feldman S. Paraventricular nucleus neuronal responses following electrical stimulation of the midbrain dorsal raphe: evidence for cotransmission. Exp Brain Res. 1989;78(2):407–414. doi: 10.1007/BF00228913. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Steinbusch H. W., Verhofstad A. A. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983 Oct 31;277(2):355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Seasholtz A. F., Thompson R. C., Douglass J. O. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988 Dec;2(12):1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Hou-Yu A., Chen W. P. Corticotropin-releasing factor synapses within the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 1989 Mar;49(3):291–299. doi: 10.1159/000125131. [DOI] [PubMed] [Google Scholar]
- Spengler D., Rupprecht R., Van L. P., Holsboer F. Identification and characterization of a 3',5'-cyclic adenosine monophosphate-responsive element in the human corticotropin-releasing hormone gene promoter. Mol Endocrinol. 1992 Nov;6(11):1931–1941. doi: 10.1210/mend.6.11.1480179. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Lind R. W., Rho J. H. The CRH motoneuron: differential peptide regulation in neurons with possible synaptic, paracrine, and endocrine outputs. Ann N Y Acad Sci. 1987;512:12–23. doi: 10.1111/j.1749-6632.1987.tb24948.x. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Watson M. A., Milbrandt J. The NGFI-B gene, a transcriptionally inducible member of the steroid receptor gene superfamily: genomic structure and expression in rat brain after seizure induction. Mol Cell Biol. 1989 Oct;9(10):4213–4219. doi: 10.1128/mcb.9.10.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]