Abstract
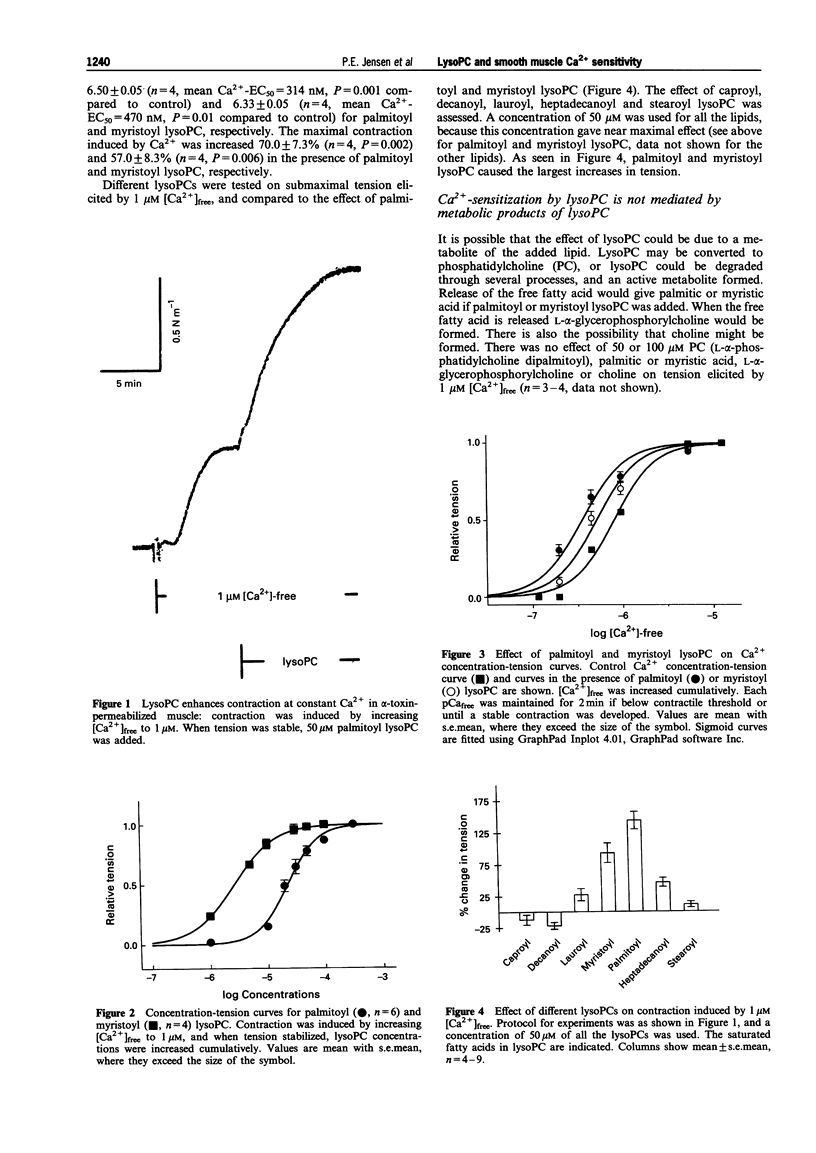
1. Pharmacological characterization of different lysophosphatidylcholines was performed based on their effect on the Ca2+ sensitivity of contraction in alpha-toxin-permeabilized rat mesenteric arteries. Furthermore, the effect of noradrenaline on [3H]-myristate-labelled lysophosphatidylcholine levels was assessed, to investigate whether lysophosphatidylcholines could be second messengers. 2. Palmitoyl or myristoyl L-alpha-lysophosphatidylcholine increased the sensitivity to Ca2+, whereas lysophosphatidylcholines containing other fatty acids had less or no effect. 3. L-alpha-phosphatidylcholine, L-alpha-glycerophosphorylcholine, palmitic acid, myristic acid and choline, potential metabolites of lysophosphatidylcholines, did not affect contractions. 4. Noradrenaline (GTP was required) and GTP gamma S increased the sensitivity to Ca2+, and GDP-beta-S inhibited the effect of noradrenaline. Lysophosphatidylcholines, however, had no requirement for GTP and caused sensitization in the presence of GDP-beta-S. 5. Calphostin C, a relatively specific protein kinase C inhibitor, did not affect contraction induced by Ca2+, but abolished the sensitizing effect of lysophosphatidylcholine. 6. Noradrenaline caused no measurable changes in the levels of [3H]-myristate-labelled phosphatidylcholine and lysophosphatidylcholine at 30 s and 5 min stimulation. 7. These results suggest that lysophosphatidylcholines can increase Ca2+ sensitivity through a G-protein-independent, but a protein kinase C-dependent mechanism. However, the role for lysophosphatidylcholines as messengers causing Ca2+ sensitization during stimulation with noradrenaline remains uncertain because no increase in [3H]-myristate labelled lysophosphatidylcholine could be measured during noradrenaline stimulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaoka Y., Oka M., Yoshida K., Sasaki Y., Nishizuka Y. Role of lysophosphatidylcholine in T-lymphocyte activation: involvement of phospholipase A2 in signal transduction through protein kinase C. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6447–6451. doi: 10.1073/pnas.89.14.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov V., Tkachuk V., Buhler F., Resink T. Phosphoinositide and calcium signalling responses in smooth muscle cells: comparison between lipoproteins, Ang II, and PDGF. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1295–1304. doi: 10.1016/0006-291x(92)91372-w. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaTorre S. D., Creer M. H., Pogwizd S. M., Corr P. B. Amphipathic lipid metabolites and their relation to arrhythmogenesis in the ischemic heart. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):11–22. doi: 10.1016/0022-2828(91)90019-i. [DOI] [PubMed] [Google Scholar]
- Ford D. A., Gross R. W. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci U S A. 1989 May;86(10):3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle J., Bassenge E., Busse R. Oxidized low density lipoproteins potentiate vasoconstrictions to various agonists by direct interaction with vascular smooth muscle. Circ Res. 1990 May;66(5):1287–1293. doi: 10.1161/01.res.66.5.1287. [DOI] [PubMed] [Google Scholar]
- Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992 Oct 25;267(30):21492–21498. [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Isocratic high-performance liquid chromatography separation of phosphoglycerides and lysophosphoglycerides. J Chromatogr. 1980 Sep 5;197(1):79–85. doi: 10.1016/s0021-9673(00)80538-5. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Armstrong M. L., Marcus M. L., Piegors D. J., Mark A. L. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984 Jun;54(6):711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991 Jan 25;266(3):1708–1715. [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman J. J., Brown K. A., Ramanadham S., Turk J., Gross R. W. Arachidonic acid release from aortic smooth muscle cells induced by [Arg8]vasopressin is largely mediated by calcium-independent phospholipase A2. J Biol Chem. 1993 Oct 5;268(28):20713–20716. [PubMed] [Google Scholar]
- McHowat J., Corr P. B. Thrombin-induced release of lysophosphatidylcholine from endothelial cells. J Biol Chem. 1993 Jul 25;268(21):15605–15610. [PubMed] [Google Scholar]
- Mehta D., Gupta S., Gaur S. N., Gangal S. V., Agrawal K. P. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am Rev Respir Dis. 1990 Jul;142(1):157–161. doi: 10.1164/ajrccm/142.1.157. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Warshaw D. M. The active tension-length curve of vascular smooth muscle related to its cellular components. J Gen Physiol. 1979 Jul;74(1):85–104. doi: 10.1085/jgp.74.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Pearce P. H., Johnsen R. D., Wysocki S. J., Kakulas B. A. Muscle lipids in Duchenne muscular dystrophy. Aust J Exp Biol Med Sci. 1981 Feb;59(1):77–90. doi: 10.1038/icb.1981.4. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Lassègue B., Alexander R. W., Griendling K. K. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem J. 1994 Apr 1;299(Pt 1):197–201. doi: 10.1042/bj2990197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Rybin V., Bernhardt J., Orlov S., Bühler F. R., Tkachuk V. A. Cellular signalling by lipoproteins in cultured smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 1993 May-Jun;30(3):169–180. doi: 10.1159/000158992. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Activation of phospholipase A2 by endothelin in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):279–286. doi: 10.1016/s0006-291x(89)80209-8. [DOI] [PubMed] [Google Scholar]
- Ruzycky A. L., Morgan K. G. Involvement of the protein kinase C system in calcium-force relationships in ferret aorta. Br J Pharmacol. 1989 Jun;97(2):391–400. doi: 10.1111/j.1476-5381.1989.tb11966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachinidis A., Locher R., Mengden T., Steiner A., Vetter W. Vasoconstriction: a novel activity for low density lipoprotein. Biochem Biophys Res Commun. 1989 Aug 30;163(1):315–320. doi: 10.1016/0006-291x(89)92137-2. [DOI] [PubMed] [Google Scholar]
- Shaikh N. A. Assessment of various techniques for the quantitative extraction of lysophospholipids from myocardial tissues. Anal Biochem. 1994 Feb 1;216(2):313–321. doi: 10.1006/abio.1994.1047. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Tomoike H., Nabeyama S., Yamamoto H., Araki H., Nakamura M., Ishii Y., Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983 Aug 5;221(4610):560–562. doi: 10.1126/science.6408736. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stoll L. L., Spector A. A. Lysophosphatidylcholine causes cGMP-dependent verapamil-sensitive Ca2+ influx in vascular smooth muscle cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C885–C893. doi: 10.1152/ajpcell.1993.264.4.C885. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Vesterqvist O., Sargent C. A., Taylor S. C., Newburger J., Tymiak A. A., Grover G. J., Ogletree M. L. Quantitation of lysophosphatidylcholine molecular species in rat cardiac tissue. Anal Biochem. 1992 Jul;204(1):72–78. doi: 10.1016/0003-2697(92)90141-s. [DOI] [PubMed] [Google Scholar]
- Ward D. T., Ohanian J., Heagerty A. M., Ohanian V. Phospholipase D-induced phosphatidate production in intact small arteries during noradrenaline stimulation: involvement of both G-protein and tyrosine-phosphorylation-linked pathways. Biochem J. 1995 Apr 15;307(Pt 2):451–456. doi: 10.1042/bj3070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. A., Insel P. A. Intracellular Ca2+ and protein kinase C interact to regulate alpha 1-adrenergic- and bradykinin receptor-stimulated phospholipase A2 activation in Madin-Darby canine kidney cells. J Biol Chem. 1991 Feb 5;266(4):2126–2133. [PubMed] [Google Scholar]
- Weisser B., Locher R., de Graaf J., Vetter W. Low density lipoprotein subfractions and [Ca2+]i in vascular smooth muscle cells. Circ Res. 1993 Jul;73(1):118–124. doi: 10.1161/01.res.73.1.118. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Hirata K., Miyake R., Akita H., Ishikawa Y., Fukuzaki H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun. 1990 Apr 16;168(1):301–308. doi: 10.1016/0006-291x(90)91708-z. [DOI] [PubMed] [Google Scholar]
- Yousufzai S. Y., Abdel-Latif A. A. Involvement of a pertussis toxin-sensitive G protein-coupled phospholipase A2 in agonist-stimulated arachidonic acid release in membranes isolated from bovine iris sphincter smooth muscle. Membr Biochem. 1993 Jan-Mar;10(1):29–42. doi: 10.3109/09687689309150250. [DOI] [PubMed] [Google Scholar]


