Abstract
1. Guanidines, amidines, S-alkylisothioureas, and recently, mercaptoalkylguanidines have been described as inhibitors of the generation of nitric oxide (NO) from L-arginine by NO synthases (NOS). We have recently demonstrated that guanidinoethyldisulphide (GED), formed from the dimerisation of mercaptoethylguanidine (MEG), is a novel inhibitor of nitric oxide synthases. Here we describe the pharmacological properties of GED on purified NOS isoforms, various cultured cell types, vascular ring preparations, and in endotoxin shock. 2. GED potently inhibited NOS activity of purified inducible NOS (iNOS), endothelial NOS (ecNOS), and brain NOS (bNOS) enzymes with Ki values of 4.3, 18 and 25 microM, respectively. Thus, GED has a 4 fold selectivity for iNOS over ecNOS at the enzyme level. The inhibitory effect of GED on ecNOS and iNOS was competitive vs. L-arginine and non-competitive vs. tetrahydrobiopterin. 3. Murine J774 macrophages, rat aortic smooth muscle cells, murine lung epithelial cells, and human intestinal DLD-1 cells were stimulated with appropriate mixtures of pro-inflammatory cytokines or bacterial lipopolysaccharide to express iNOS. In these cells, GED potently inhibited nitrite formation (EC50 values: 11, 9, 1 and 30 microM, respectively). This suggests that uptake of GED may be cell type and species-dependent. The inhibitory effect of GED on nitrite production was independent of whether GED was given together with immunostimulation or 6 h afterwards, indicating that GED does not interfere with the process of iNOS induction. 4. GED caused relaxations in the precontracted vascular ring preparations (EC50: 20 microM). Part of this relaxation was endothelium-dependent, but was not blocked by methylene blue (100 microM), an inhibitor of soluble guanylyl cyclase. In precontracted rings, GED enhanced the acetylcholine-induced, endothelium-dependent relaxations at 10 microM and caused a slight inhibition of the relaxations at 100 microM. The vascular studies demonstrate that the inhibitory potency of GED on ecNOS in the ring preparations is considerably lower than its potency against iNOS in the cultured cells. These data suggest that the selectivity of GED towards iNOS may lie, in part, at the enzyme level, as well as differential uptake by cells expressing the various isoforms of NOS. 5. In a rat model of endotoxin shock in vivo, administration of GED, at 3 mg kg-1 bolus followed by 10 mg kg-1 h-1 infusion, starting at 90 min after bacterial lipopolysaccharide (LPS, 15 mg kg-1, i.v.), prevented the delayed fall in mean arterial blood pressure, prevented the development of the vascular hyporeactivity to noradrenaline of the thoracic aorta ex vivo and protected against the impairment of the endothelium-dependent relaxations associated with this model of endotoxaemia. The same bolus and infusion of the inhibitor did not alter blood pressure or ex vivo vascular reactivity in normal animals over 90 min. 6. Administration of GED (10 mg kg-1, i.p.) given at 2 h after LPS (120 mg kg-1, i.p.) and every 6 h thereafter caused a significant improvement in the survival rate in a lethal model of endotoxin shock in mice between 12 and 42 h. 7. In conclusion, we found that GED is a competitive inhibitor of iNOS activity. Its selectivity towards iNOS may lie both at the enzyme level and at the level of cell uptake. GED has beneficial effects in models of endotoxin shock that are driven by iNOS. GED or its derivatives may be useful tools in the experimental therapy of inflammatory conditions associated with NO overproduction due to iNOS expression.
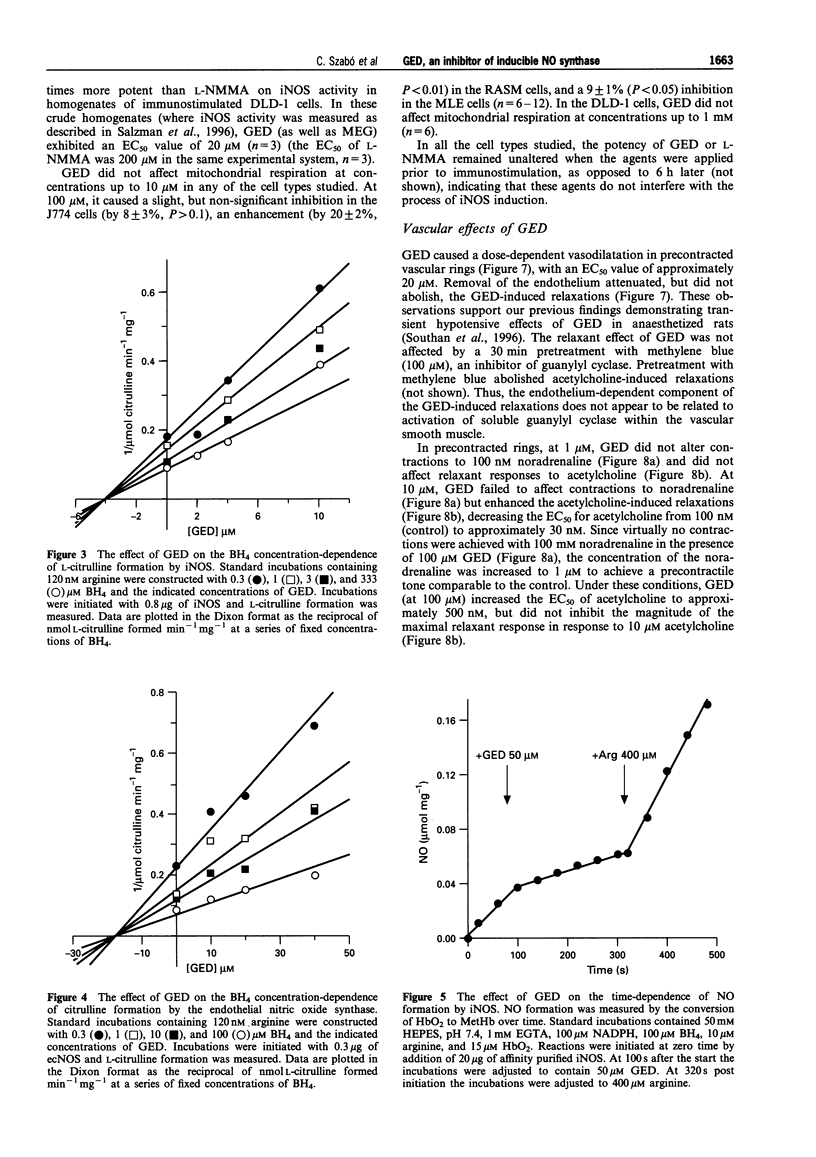
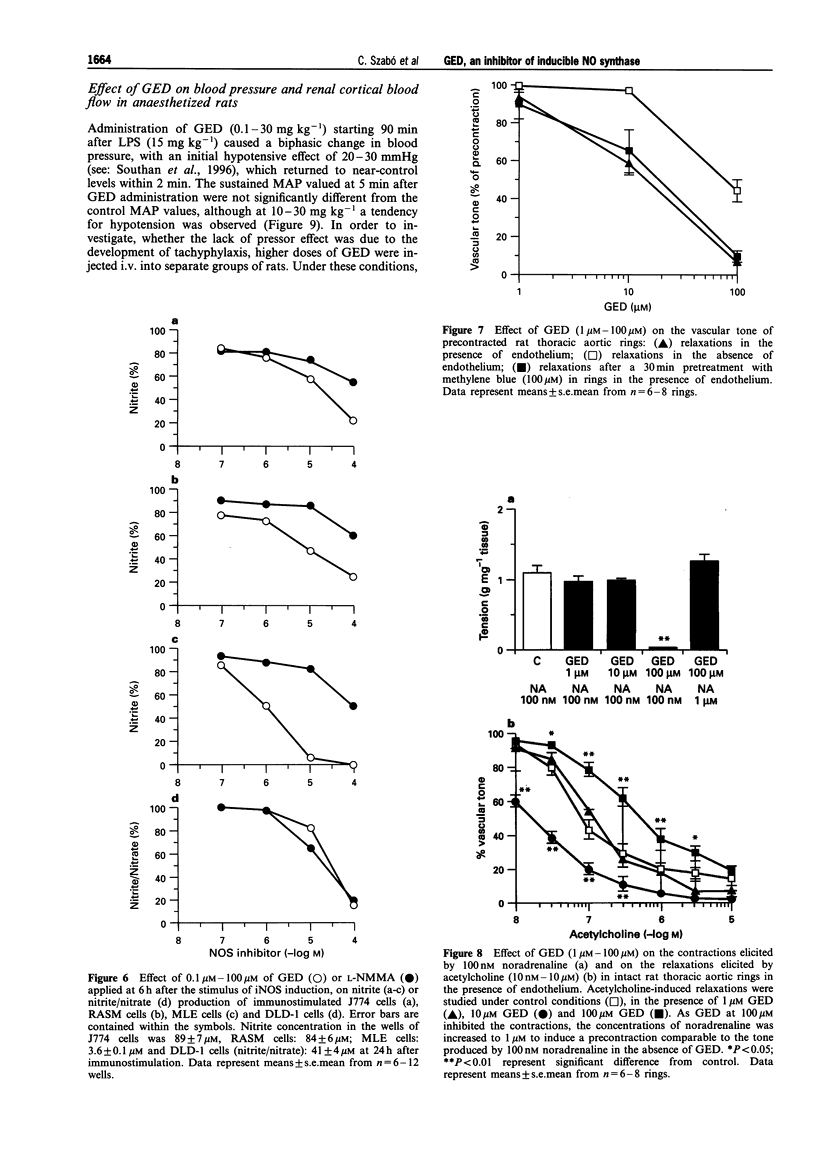
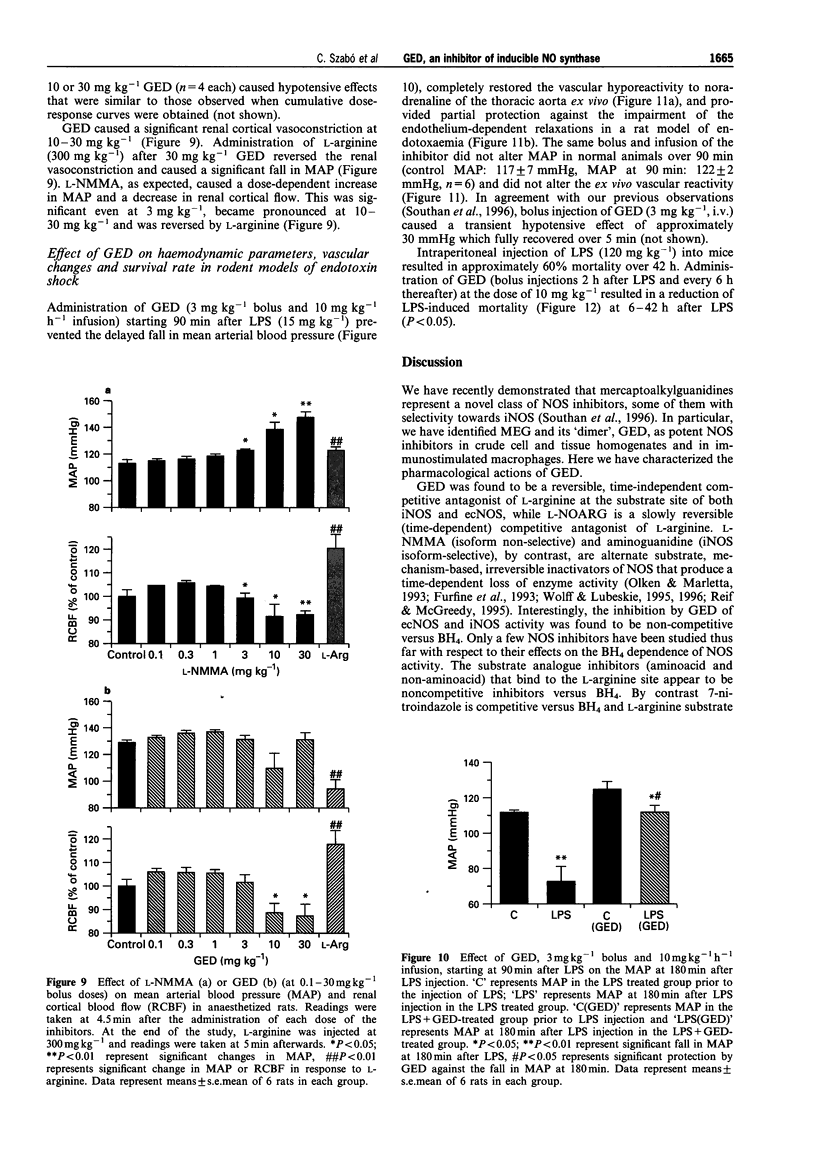
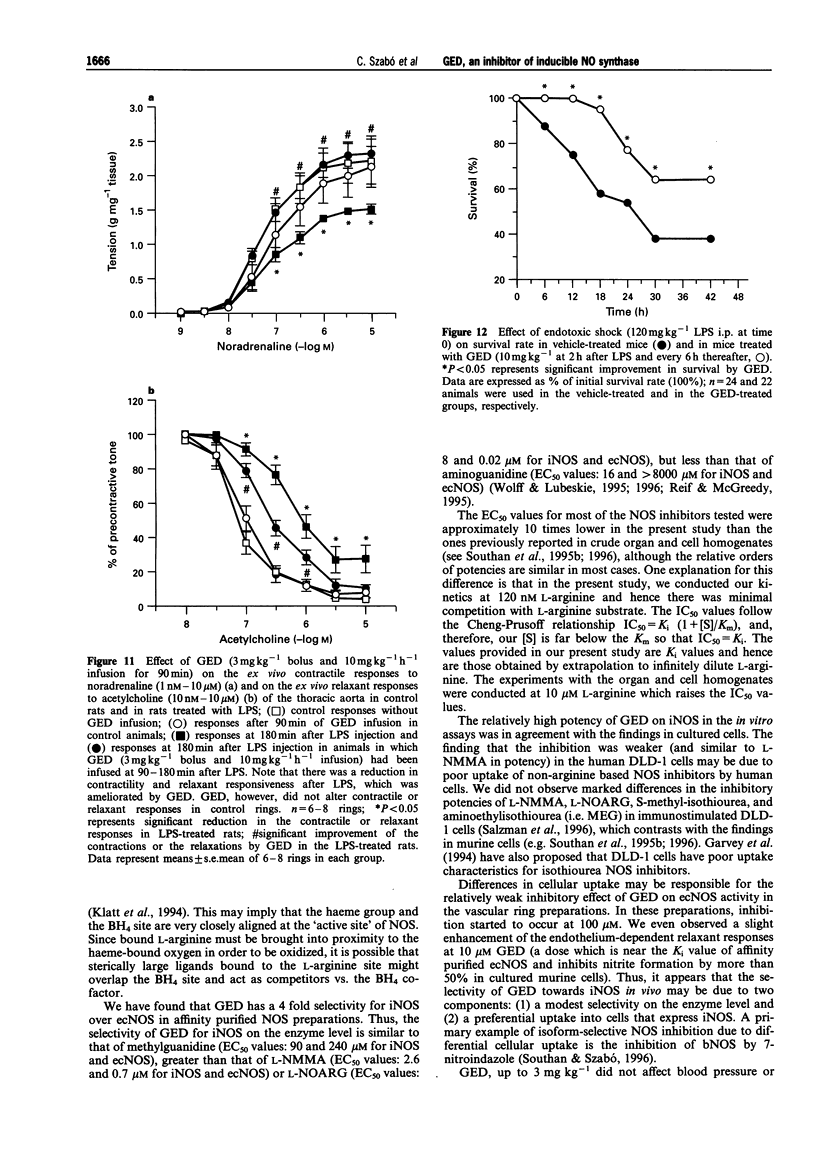
Full text
PDF




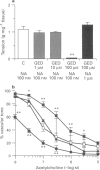
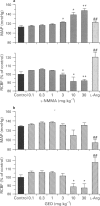
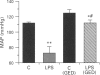
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY D. G., BURNETT W. T., Jr, SHAPIRA R. Chemical protection against ionizing radiation. II. Mercaptoalkylamines and related compounds with protective activity. Radiat Res. 1957 Jul;7(1):13–21. [PubMed] [Google Scholar]
- Furfine E. S., Harmon M. F., Paith J. E., Garvey E. P. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993 Aug 24;32(33):8512–8517. doi: 10.1021/bi00084a017. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Oplinger J. A., Tanoury G. J., Sherman P. A., Fowler M., Marshall S., Harmon M. F., Paith J. E., Furfine E. S. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J Biol Chem. 1994 Oct 28;269(43):26669–26676. [PubMed] [Google Scholar]
- Gross S. S., Stuehr D. J., Aisaka K., Jaffe E. A., Levi R., Griffith O. W. Macrophage and endothelial cell nitric oxide synthesis: cell-type selective inhibition by NG-aminoarginine, NG-nitroarginine and NG-methylarginine. Biochem Biophys Res Commun. 1990 Jul 16;170(1):96–103. doi: 10.1016/0006-291x(90)91245-n. [DOI] [PubMed] [Google Scholar]
- Hasan K., Heesen B. J., Corbett J. A., McDaniel M. L., Chang K., Allison W., Wolffenbuttel B. H., Williamson J. R., Tilton R. G. Inhibition of nitric oxide formation by guanidines. Eur J Pharmacol. 1993 Nov 2;249(1):101–106. doi: 10.1016/0014-2999(93)90667-7. [DOI] [PubMed] [Google Scholar]
- KOLLMANN G., SHAPIRO B., SCHWARTZ E. E. THE MECHANISM OF ACTION OF AET. V. THE DISTRIBUTION AND THE CHEMICAL FORMS OF 2-MERCAPTOETHYLGUANIDINE AND BIS (2-GUANIDOETHYL) DISULFIDE GIVEN ORALLY IN PROTECTIVE DOSES TO MICE. Radiat Res. 1963 Sep;20:17–23. [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Brunner F., Mayer B. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J Biol Chem. 1994 Jan 21;269(3):1674–1680. [PubMed] [Google Scholar]
- Kozák I., Arient M. Metabolism and radioprotective effect of AET and MEG in organism. Strahlentherapie. 1973 Sep;146(3):367–373. [PubMed] [Google Scholar]
- Lambert L. E., Whitten J. P., Baron B. M., Cheng H. C., Doherty N. S., McDonald I. A. Nitric oxide synthesis in the CNS endothelium and macrophages differs in its sensitivity to inhibition by arginine analogues. Life Sci. 1991;48(1):69–75. doi: 10.1016/0024-3205(91)90426-c. [DOI] [PubMed] [Google Scholar]
- MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995 May 19;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Olken N. M., Marletta M. A. NG-methyl-L-arginine functions as an alternate substrate and mechanism-based inhibitor of nitric oxide synthase. Biochemistry. 1993 Sep 21;32(37):9677–9685. doi: 10.1021/bi00088a020. [DOI] [PubMed] [Google Scholar]
- Reif D. W., McCreedy S. A. N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch Biochem Biophys. 1995 Jun 20;320(1):170–176. doi: 10.1006/abbi.1995.1356. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ E. E., SHAPIRO B. The protection of mice against radiation by 2-mercaptoethylguanidine and its disulfide. Radiat Res. 1960 Nov;13:768–775. [PubMed] [Google Scholar]
- SHAPIRO B., SCHWARTZ E. E., KOLLMANN G. The mechanism of action of AET. IV. The distribution and the chemical forms of 2-mercaptoethylguanidine and bis(2-guanidoethyl) disulfide in protected mice. Radiat Res. 1963 Jan;18:17–30. [PubMed] [Google Scholar]
- Southan G. J., Szabó C., Connor M. P., Salzman A. L., Thiemermann C. Amidines are potent inhibitors of nitric oxide synthases: preferential inhibition of the inducible isoform. Eur J Pharmacol. 1995 Nov 30;291(3):311–318. doi: 10.1016/0922-4106(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Southan G. J., Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996 Feb 23;51(4):383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Southan G. J., Szabó C., Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995 Jan;114(2):510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan G. J., Zingarelli B., O'Connor M., Salzman A. L., Szabó C. Spontaneous rearrangement of aminoalkylisothioureas into mercaptoalkylguanidines, a novel class of nitric oxide synthase inhibitors with selectivity towards the inducible isoform. Br J Pharmacol. 1996 Feb;117(4):619–632. doi: 10.1111/j.1476-5381.1996.tb15236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 1995 Feb;3(1):2–32. [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1993 May;265(2):674–680. [PubMed] [Google Scholar]
- Szabó C., Salzman A. L., Ischiropoulos H. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 1995 Apr 24;363(3):235–238. doi: 10.1016/0014-5793(95)00322-z. [DOI] [PubMed] [Google Scholar]
- Szabó C., Southan G. J., Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Zingarelli B., O'Connor M., Salzman A. L. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa L. M., Salas E., Darley-Usmar V. M., Radomski M. W., Moncada S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Schaffner A., Schoedon G. Differential regulation of constitutive and inducible nitric oxide production by inflammatory stimuli in murine endothelial cells. Biochem Biophys Res Commun. 1994 Jul 15;202(1):450–455. doi: 10.1006/bbrc.1994.1949. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Wikenheiser K. A., Vorbroker D. K., Rice W. R., Clark J. C., Bachurski C. J., Oie H. K., Whitsett J. A. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Datto G. A. Identification and characterization of a calmodulin-dependent nitric oxide synthase from GH3 pituitary cells. Biochem J. 1992 Jul 1;285(Pt 1):201–206. doi: 10.1042/bj2850201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Gribin B. J. The inhibition of the constitutive and inducible nitric oxide synthase isoforms by indazole agents. Arch Biochem Biophys. 1994 Jun;311(2):300–306. doi: 10.1006/abbi.1994.1241. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Biochem Biophys. 1995 Jan 10;316(1):290–301. doi: 10.1006/abbi.1995.1040. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Lubeskie A. Inactivation of nitric oxide synthase isoforms by diaminoguanidine and NG-amino-L-arginine. Arch Biochem Biophys. 1996 Jan 15;325(2):227–234. doi: 10.1006/abbi.1996.0028. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Lubeskie A., Umansky S. The inhibition of the constitutive bovine endothelial nitric oxide synthase by imidazole and indazole agents. Arch Biochem Biophys. 1994 Nov 1;314(2):360–366. doi: 10.1006/abbi.1994.1454. [DOI] [PubMed] [Google Scholar]
- Worrall N. K., Lazenby W. D., Misko T. P., Lin T. S., Rodi C. P., Manning P. T., Tilton R. G., Williamson J. R., Ferguson T. B., Jr Modulation of in vivo alloreactivity by inhibition of inducible nitric oxide synthase. J Exp Med. 1995 Jan 1;181(1):63–70. doi: 10.1084/jem.181.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Chen S. J., Szabó C., Thiemermann C., Vane J. R. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995 Apr;114(8):1666–1672. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B., Caputi A. P., Di Rosa M. Dexamethasone prevents vascular failure mediated by nitric oxide in hemorrhagic shock. Shock. 1994 Sep;2(3):210–215. doi: 10.1097/00024382-199409000-00009. [DOI] [PubMed] [Google Scholar]