Abstract
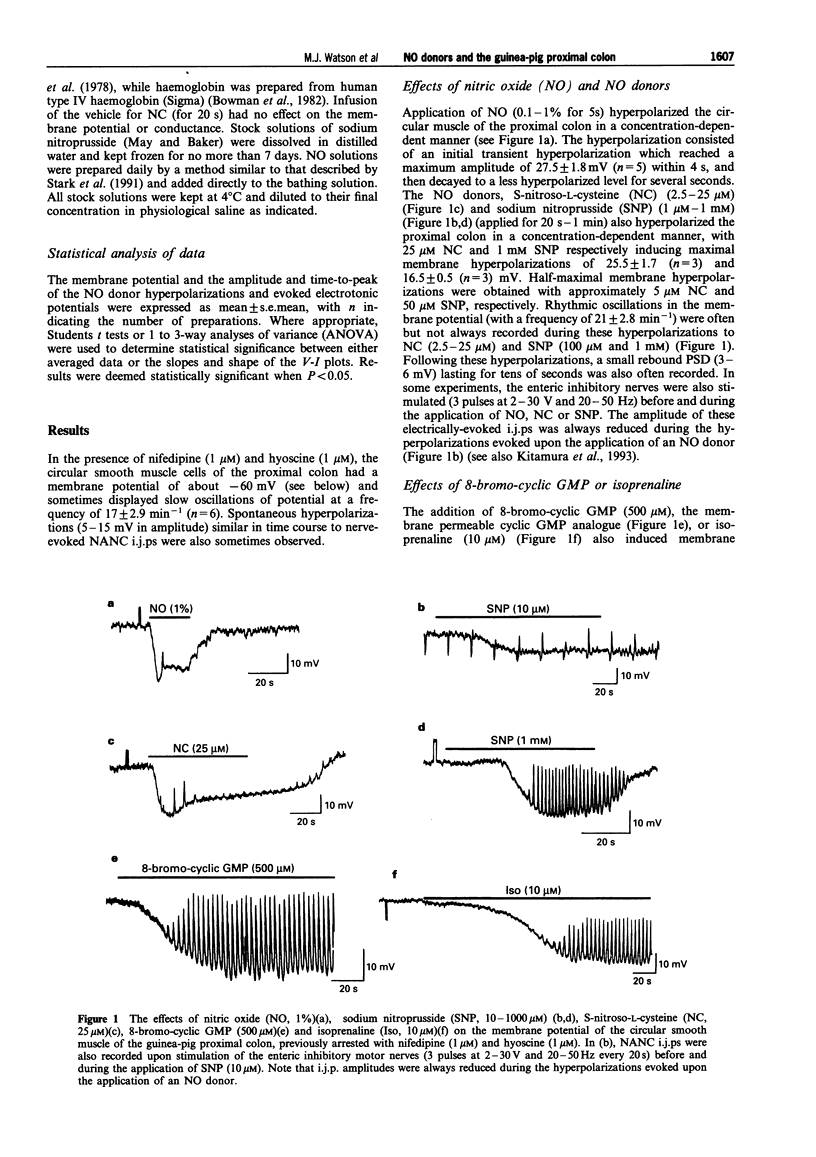
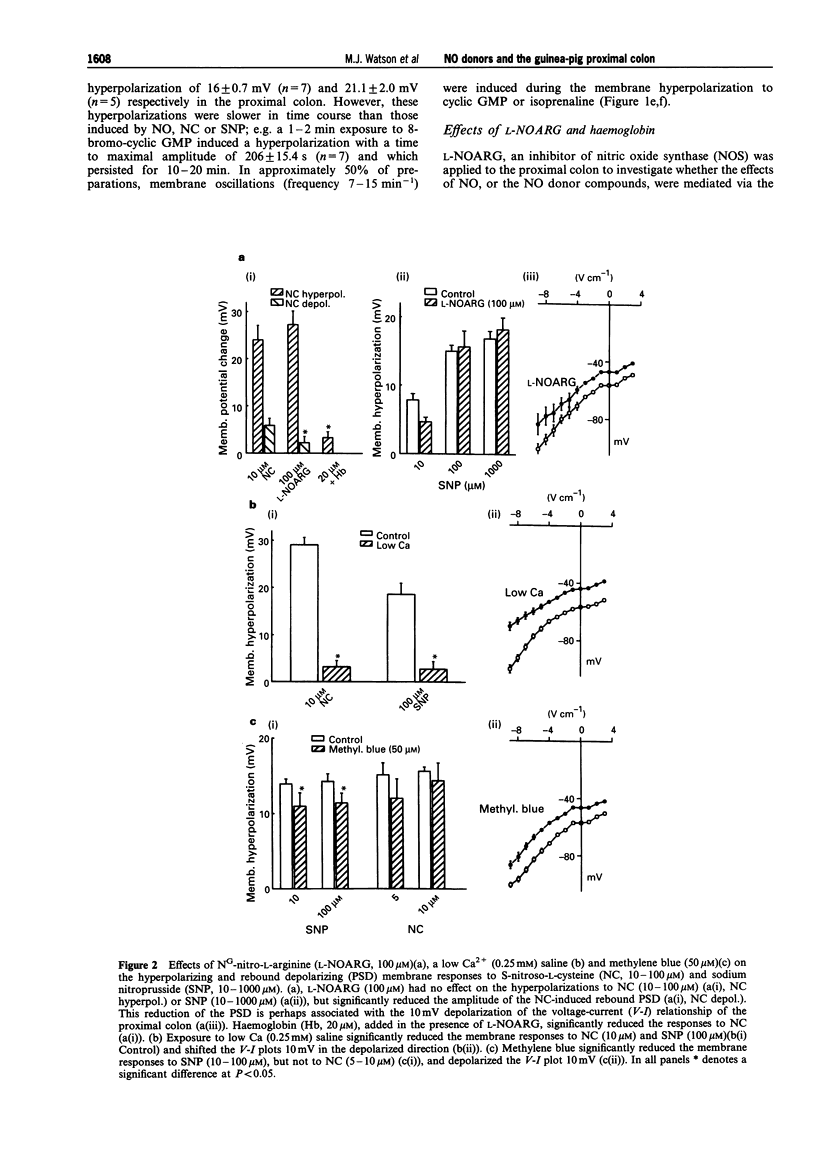
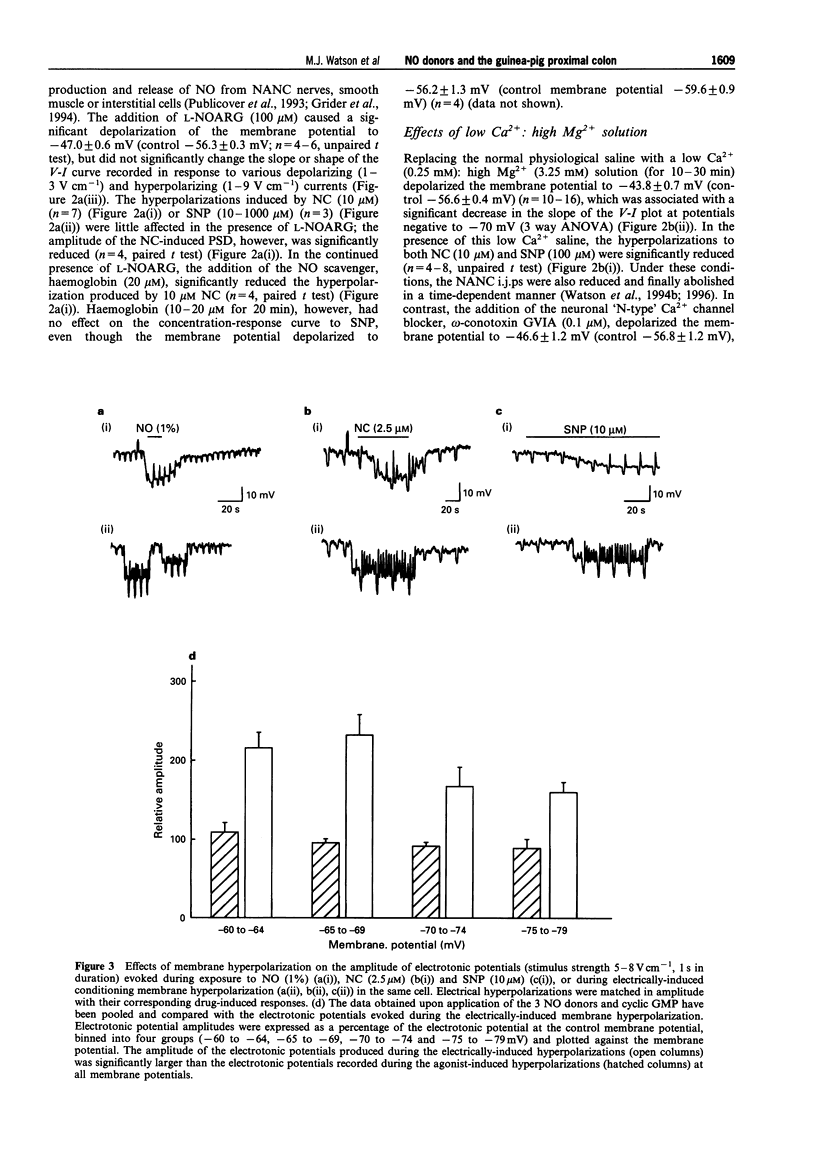
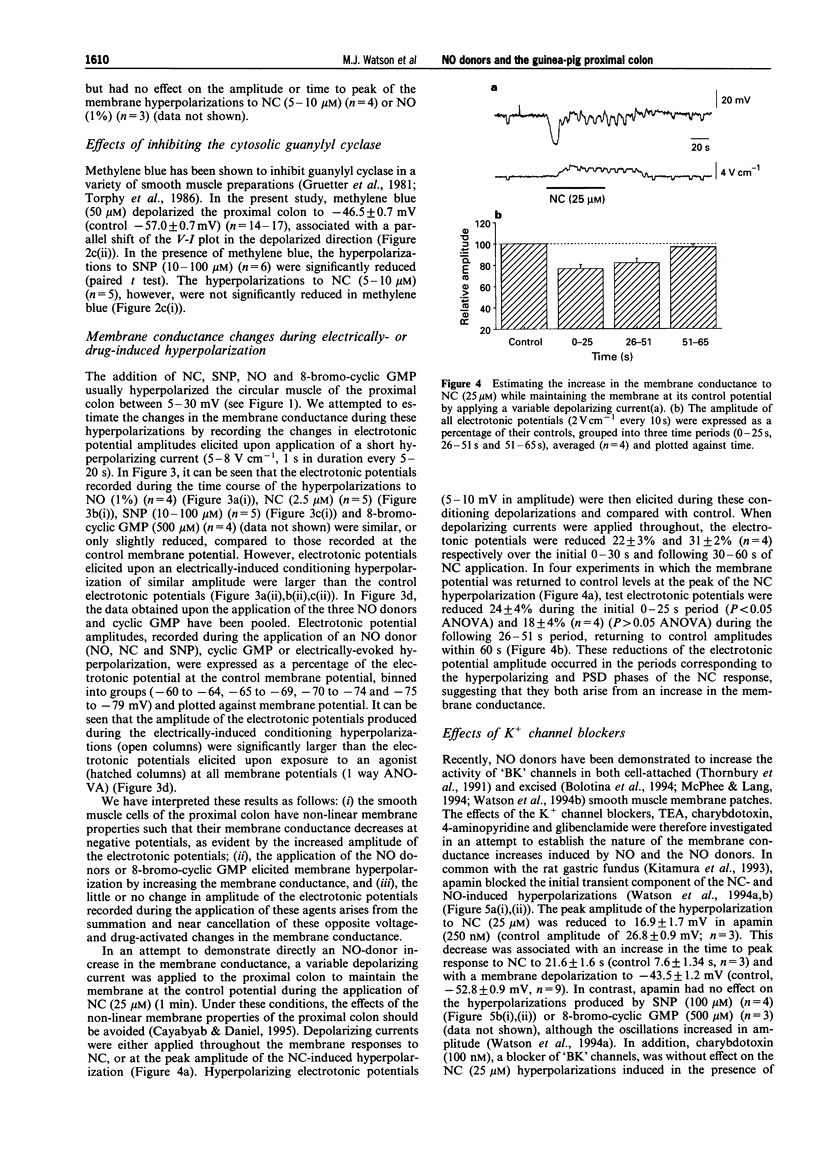
1. The membrane conductance changes underlying the membrane hyperpolarizations induced by nitric oxide (NO), S-nitroso-L-cysteine (NC) and sodium nitroprusside (SNP) were investigated in the circular smooth muscle cells of the guinea-pig proximal colon, by use of standard intracellular microelectrode recording techniques. 2. NO (1%), NC (2.5-25 microM) and SNP (1-1000 microM) induced membrane hyperpolarization in a concentration-dependent manner, the hyperpolarizations to NO and NC developing more rapidly than those to SNP. The slower-developing responses to SNP were mimicked by the membrane permeable analogue of guanosine 3':5' cyclic-monophosphate (cyclic GMP), 8-bromo-cyclic GMP (500 microM), and by isoprenaline (10 microM). 3. The hyperpolarizations to NC and SNP were reduced in a low Ca2+ (0.25 mM) saline and upon the addition of haemoglobin (20 microM), but were not effected by NG-nitro-L-arginine (L-NOARG) (100 microM) or omega-conotoxin GVIA (100 nM). the hyperpolarizations to SNP were also significantly reduced by methylene blue (50 microM). 4. Apamin (250 nM) depolarized the membrane potential approximately 10 mV and reduced the initial transient component of the hyperpolarization to NO (1%) and NC (25 microM), but had no effects on the hyperpolarizations to SNP and cyclic GMP. Tetraethylammonium (TEA) (5-15 mM), had little effect on the membrane responses to NO(1%), NC(2.5-25 microM), SNP(100(-1000) microM) or cyclic GMP(500 microM). However, TEA (5-15 mM) reduced the membrane hyperpolarizations to SNP (10 microM) and isoprenaline (10 microM) in a concentration-dependent manner. The hyperpolarization to isoprenaline (10 microM) remaining in the presence of 15 mM TEA was blocked by ouabain (10 microM). 5. The amplitude of electronic potentials (1 s duration) elicited during NO donor hyperpolarizations were little changed or only slightly reduced (5-25%). However, the amplitude of the electrotonic potentials elicited during maintained electrically-induced hyperpolarizations of similar amplitude were significantly increased (30-150%), suggesting that the non-linear membrane properties of the proximal colon partially mask an increase in membrane conductance elicited during the NO donor hyperpolarizations. 6. Membrane hyperpolarization in the presence of an NO donor, 8-bromo-cyclic GMP, isoprenaline, or upon application of a maintained hyperpolarizing electrical current, often evoked oscillations of the membrane potential. These oscillations were prevented by Cs+ (1 mM). 7. These results indicate that NO and NC hyperpolarize the circular muscle of the proximal colon by activating at least two TEA-resistant membrane K+ conductances, one of which is sensitive to apamin blockade. The K+ conductance increases activated by SNP or 8-bromo-cyclic GMP were little effected by apamin, perhaps suggesting a common mechanism. In contrast, the hyperpolarization to isoprenaline appears to involve the activation of TEA-sensitive Ca2(+)-activated K+ ('BK') channels, as well as a Na:K ATPase. Finally, the 'background' membrane conductance of the circular muscle cells of the proximal colon decreased upon membrane hyperpolarization to reveal oscillations of the membrane potential which may well represent 'pacemaker' or 'slow wave' activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayguinov O., Sanders K. M. Role of nitric oxide as an inhibitory neurotransmitter in the canine pyloric sphincter. Am J Physiol. 1993 May;264(5 Pt 1):G975–G983. doi: 10.1152/ajpgi.1993.264.5.G975. [DOI] [PubMed] [Google Scholar]
- Bayguinov O., Vogalis F., Morris B., Sanders K. M. Patterns of electrical activity and neural responses in canine proximal duodenum. Am J Physiol. 1992 Dec;263(6 Pt 1):G887–G894. doi: 10.1152/ajpgi.1992.263.6.G887. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. Non-cholinergic fast and slow post-stimulus depolarization in the guinea-pig ileum. J Physiol. 1983 Jul;340:47–56. doi: 10.1113/jphysiol.1983.sp014748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Cayabyab F. S., Daniel E. E. K+ channel opening mediates hyperpolarizations by nitric oxide donors and IJPs in opossum esophagus. Am J Physiol. 1995 May;268(5 Pt 1):G831–G842. doi: 10.1152/ajpgi.1995.268.5.G831. [DOI] [PubMed] [Google Scholar]
- Christinck F., Jury J., Cayabyab F., Daniel E. E. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 1991 Oct;69(10):1448–1458. doi: 10.1139/y91-217. [DOI] [PubMed] [Google Scholar]
- Crist J. R., He X. D., Goyal R. K. Chloride-mediated junction potentials in circular muscle of the guinea pig ileum. Am J Physiol. 1991 Nov;261(5 Pt 1):G742–G751. doi: 10.1152/ajpgi.1991.261.5.G742. [DOI] [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Helmy-Elkholy A., Jager L. P., Kannan M. S. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983 Mar;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Murray J., Bates J. N., Conklin J. L. Nitric oxide: mediator of NANC hyperpolarization of opossum esophageal smooth muscle. Am J Physiol. 1991 Dec;261(6 Pt 1):G1012–G1016. doi: 10.1152/ajpgi.1991.261.6.G1012. [DOI] [PubMed] [Google Scholar]
- Furness J. B. The presence of inhibitory nerves in the colon after sympathetic denervation. Eur J Pharmacol. 1969;6(3):349–352. doi: 10.1016/0014-2999(69)90196-4. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Lecci A., Giachetti A., Maggi C. A. Tachykinins and reflexly evoked atropine-resistant motility in the guinea pig colon in vivo. J Pharmacol Exp Ther. 1993 Jun;265(3):1224–1231. [PubMed] [Google Scholar]
- Grider J. R., Murthy K. S., Jin J. G., Makhlouf G. M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992 Apr;262(4 Pt 1):G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Kadowitz P. J., Ignarro L. J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981 Feb;59(2):150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- Jury J., Ahmedzadeh N., Daniel E. E. A mediator derived from arginine mediates inhibitory junction potentials and relaxations in lower esophageal sphincter: an independent role for vasoactive intestinal peptide. Can J Physiol Pharmacol. 1992 Aug;70(8):1182–1189. doi: 10.1139/y92-164. [DOI] [PubMed] [Google Scholar]
- Jury J., Jager L. P., Daniel E. E. Unusual potassium channels mediate nonadrenergic noncholinergic nerve-mediated inhibition in opossum esophagus. Can J Physiol Pharmacol. 1985 Feb;63(2):107–112. doi: 10.1139/y85-020. [DOI] [PubMed] [Google Scholar]
- Keef K. D., Du C., Ward S. M., McGregor B., Sanders K. M. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993 Oct;105(4):1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Lian Q., Carl A., Kuriyama H. S-nitrosocysteine, but not sodium nitroprusside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br J Pharmacol. 1993 Jun;109(2):415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Lyster D. J., Bywater R. A., Taylor G. S., Watson M. J. Effects of a nitric oxide synthase inhibitor on non-cholinergic junction potentials in the circular muscle of the guinea pig ileum. J Auton Nerv Syst. 1992 Dec;41(3):187–196. doi: 10.1016/0165-1838(92)90058-o. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S. Multiple inhibitory mechanisms mediate non-adrenergic non-cholinergic relaxation in the circular muscle of the guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jun;347(6):630–634. doi: 10.1007/BF00166946. [DOI] [PubMed] [Google Scholar]
- Prosser C. L. Rhythmic potentials in intestinal muscle. Fed Proc. 1978 Jun;37(8):2153–2157. [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha J. K., Hirano I., Goyal R. K. Biphasic effect of SNP on opossum esophageal longitudinal muscle: involvement of cGMP and eicosanoids. Am J Physiol. 1993 Aug;265(2 Pt 1):G403–G407. doi: 10.1152/ajpgi.1993.265.2.G403. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Electrical pacemakers of canine proximal colon are functionally innervated by inhibitory motor neurons. Am J Physiol. 1989 Mar;256(3 Pt 1):C466–C477. doi: 10.1152/ajpcell.1989.256.3.C466. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Bauer A. J., Szurszewski J. H. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991 Dec;444:743–761. doi: 10.1113/jphysiol.1991.sp018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthamnatpong N., Hosokawa M., Takeuchi T., Hata F., Takewaki T. Nitric oxide-mediated inhibitory response of rat proximal colon: independence from changes in membrane potential. Br J Pharmacol. 1994 Jun;112(2):676–682. doi: 10.1111/j.1476-5381.1994.tb13129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol. 1972 Sep;225(3):693–703. doi: 10.1113/jphysiol.1972.sp009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Fine C. F., Burman M., Barnette M. S., Ormsbee H. S., 3rd Lower esophageal sphincter relaxation is associated with increased cyclic nucleotide content. Am J Physiol. 1986 Dec;251(6 Pt 1):G786–G793. doi: 10.1152/ajpgi.1986.251.6.G786. [DOI] [PubMed] [Google Scholar]
- Venkova K., Krier J. A nitric oxide and prostaglandin-dependent component of NANC off-contractions in cat colon. Am J Physiol. 1994 Jan;266(1 Pt 1):G40–G47. doi: 10.1152/ajpgi.1994.266.1.G40. [DOI] [PubMed] [Google Scholar]
- Vogalis F., Lang R. J., Bywater R. A., Taylor G. S. Voltage-gated ionic currents in smooth muscle cells of guinea pig proximal colon. Am J Physiol. 1993 Mar;264(3 Pt 1):C527–C536. doi: 10.1152/ajpcell.1993.264.3.C527. [DOI] [PubMed] [Google Scholar]
- Vogalis F., Lang R. J. Identification of single transiently opening ("A-type") K channels in guinea-pig colonic myocytes. Pflugers Arch. 1994 Dec;429(2):160–164. doi: 10.1007/BF00374307. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Bradley M. E., Buxton I. L., Keef K., Westfall D. P., Sanders K. M. Involvement of cyclic GMP in non-adrenergic, non-cholinergic inhibitory neurotransmission in dog proximal colon. Br J Pharmacol. 1992 Dec;107(4):1075–1082. doi: 10.1111/j.1476-5381.1992.tb13409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- Ward S. M., McKeen E. S., Sanders K. M. Role of nitric oxide in non-adrenergic, non-cholinergic inhibitory junction potentials in canine ileocolonic sphincter. Br J Pharmacol. 1992 Apr;105(4):776–782. doi: 10.1111/j.1476-5381.1992.tb09056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V., Maggi C. A. Electrophysiological evidence for different release mechanism of ATP and NO as inhibitory NANC transmitters in guinea-pig colon. Br J Pharmacol. 1994 Aug;112(4):1077–1082. doi: 10.1111/j.1476-5381.1994.tb13193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A., Jager L. P. Ion fluxes during the inhibitory junction potential in the guinea-pig taenia coli. J Physiol. 1975 Sep;250(3):681–691. doi: 10.1113/jphysiol.1975.sp011077. [DOI] [PMC free article] [PubMed] [Google Scholar]


