Abstract
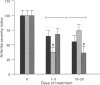
1. Currently available pharmacological therapies treat arthritis inadequately. We have previously found that the kappa (kappa)-opioid, U-50,488H (trans-(+/-)- 3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl]- benzene-acetamide methane sulphonate), possesses anti-arthritic effects. In light of the finding that opioid receptors in the periphery are upregulated during inflammation, kappa-opioids may represent a novel therapy for arthritis. The primary aim and unique feature of the present study is to investigate whether opioids exert their anti-arthritic effects in the periphery. Thus, the dose-effect relationship of a kappa-opioid agonist, U-50,488H was compared after both local and distant administration. Further, we tested whether the anti-arthritic effects of this drug are stereospecific and receptor-mediated by use of opioid antagonists. 2. Using an adjuvant model of arthritis in male Lewis rats, arthritis was judged by oedema, radiography and histological changes in the contralateral ankle of the hind limb. Treatment with (+/-)-U-50,488H for 3 days during disease onset and 3 days during established disease significantly attenuated arthritis, but the effects of (+/-)-U-50,488H on radiology and histology varied according to treatment time. Administration of (+/-)-U-50,488H during disease onset had a more marked effect on radiography, suggesting that treatment with that drug should be started early to prevent progressive joint destruction. Further, it was found that (+/-)-U-50,488H, administered for 3 days during the disease onset, either by direct subcutaneous injection into the inflamed paw or at a more distant site into the back of the neck, dose-dependently attenuated arthritic damage as measured by an index which pooled all three variables. More importantly however, (+/-)-U-50,488H was approximately fourfold more potent as an 'anti-arthritic' agent after local compared to distant subcutaneous injection (ED50; local vs distant: 5.8 +/- 1.6 vs 19.5 +/- 0.8 mg kg-1). 3. Equivalent doses of the (-)-enantiomer (20 mg kg-1day-1) and the racemate (+/-) of U-50,488H (40 mg kg-1day-1), elicited a similar attenuation of arthritic parameters while the (+/-)-enantiomer exacerbated arthritis, suggesting that the anti-arthritic activity lies solely with the (-)-enantiomer. 4. Both the peripherally selective antagonist, naloxone methiodide, and the kappa-selective antagonist, MR2266 ((-)-5,9 alpha-diethyl-2-(3-furylmethyl)-2'-hydroxy-6,7-benzomorphan), were able to reverse fully the peripheral anti-arthritic effects of U-50,488H, indicating that it exerts its effects through peripheral kappa-opioid receptors. 5. Taken together, these results not only confirm our previous findings that demonstrate anti-arthritic effects of U-50,488H but they indicate that the opioid attenuation of experimental arthritis is mediated via peripheral kappa-receptors in the arthritic joint. Peripherally acting kappa-opioid agonists should lead to new therapies for arthritis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. R., Rooks W. H., 2nd, Shott L., Genant H., Maloney P., West E. Effects of naproxen on connective tissue changes in the adjuvant arthritic rat. Arthritis Rheum. 1979 Dec;22(12):1365–1374. doi: 10.1002/art.1780221208. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Bjurholm A., Srinivasan G. R., Lundeberg T., Theodorsson E., Schultzberg M., Kreicbergs A. Capsaicin effects on substance P and CGRP in rat adjuvant arthritis. Regul Pept. 1995 Jan 5;55(1):85–102. doi: 10.1016/0167-0115(94)00095-f. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Srinivasan G. R., Theodorsson E., Schultzberg M., Kreicbergs A. Effects of surgical denervation on substance P and calcitonin gene-related peptide in adjuvant arthritis. Peptides. 1995;16(4):569–579. doi: 10.1016/0196-9781(95)00025-f. [DOI] [PubMed] [Google Scholar]
- Antonijevic I., Mousa S. A., Schäfer M., Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995 Jan;15(1 Pt 1):165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A., Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992 Sep;12(5):525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Levine J. D. Opiate analgesia. How central is a peripheral target? N Engl J Med. 1991 Oct 17;325(16):1168–1169. doi: 10.1056/NEJM199110173251610. [DOI] [PubMed] [Google Scholar]
- Carmody J. J. Opiate receptors: an introduction. Anaesth Intensive Care. 1987 Feb;15(1):27–37. doi: 10.1177/0310057X8701500106. [DOI] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Kidd B. L. Sensory denervation with capsaicin attenuates inflammation and nociception in arthritic rats. Neurosci Lett. 1995 Jul 7;193(3):205–207. doi: 10.1016/0304-3940(95)11704-z. [DOI] [PubMed] [Google Scholar]
- Członkowski A., Stein C., Herz A. Peripheral mechanisms of opioid antinociception in inflammation: involvement of cytokines. Eur J Pharmacol. 1993 Oct 5;242(3):229–235. doi: 10.1016/0014-2999(93)90246-e. [DOI] [PubMed] [Google Scholar]
- Donaldson L. F., McQueen D. S., Seckl J. R. Neuropeptide gene expression and capsaicin-sensitive primary afferents: maintenance and spread of adjuvant arthritis in the rat. J Physiol. 1995 Jul 15;486(Pt 2):473–482. doi: 10.1113/jphysiol.1995.sp020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra L. A., Gmerek D. E., Winger G., Woods J. H. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987 Aug;242(2):413–420. [PubMed] [Google Scholar]
- Ferreira S. H., Nakamura M. II - Prostaglandin hyperalgesia: the peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins. 1979 Aug;18(2):191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Opioid control of the function of primary afferent substance P fibres. Eur J Pharmacol. 1985 Aug 27;114(3):241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- Leon A., Buriani A., Dal Toso R., Fabris M., Romanello S., Aloe L., Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Collier D. H., Basbaum A. I., Moskowitz M. A., Helms C. A. Hypothesis: the nervous system may contribute to the pathophysiology of rheumatoid arthritis. J Rheumatol. 1985 Jun;12(3):406–411. [PubMed] [Google Scholar]
- Levine J. D., Goetzl E. J., Basbaum A. I. Contribution of the nervous system to the pathophysiology of rheumatoid arthritis and other polyarthritides. Rheum Dis Clin North Am. 1987 Aug;13(2):369–383. [PubMed] [Google Scholar]
- Levine J. D., Moskowitz M. A., Basbaum A. I. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985 Aug;135(2 Suppl):843s–847s. [PubMed] [Google Scholar]
- Millan M. J., Colpaert F. C. Opioid systems in the response to inflammatory pain: sustained blockade suggests role of kappa- but not mu-opioid receptors in the modulation of nociception, behaviour and pathology. Neuroscience. 1991;42(2):541–553. doi: 10.1016/0306-4522(91)90396-6. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Brantl V., Herz A., Emrich H. M. Psychotomimesis mediated by kappa opiate receptors. Science. 1986 Aug 15;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pincus T., Marcum S. B., Callahan L. F. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol. 1992 Dec;19(12):1885–1894. [PubMed] [Google Scholar]
- Pincus T. Rheumatoid arthritis: a medical emergency? Scand J Rheumatol Suppl. 1994;100:21–30. doi: 10.3109/03009749409095198. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., France C. P., Bykov V., De Costa B. R., Jacobson A. E., Woods J. H., Rice K. C. Pharmacological activities of optically pure enantiomers of the kappa opioid agonist, U50,488, and its cis diastereomer: evidence for three kappa receptor subtypes. Eur J Pharmacol. 1989 Aug 29;167(3):345–353. doi: 10.1016/0014-2999(89)90443-3. [DOI] [PubMed] [Google Scholar]
- Sibinga N. E., Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- Stein C., Comisel K., Haimerl E., Yassouridis A., Lehrberger K., Herz A., Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991 Oct 17;325(16):1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Przewłocki R., Gramsch C., Peter K., Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Millan M. J., Shippenberg T. S., Peter K., Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989 Mar;248(3):1269–1275. [PubMed] [Google Scholar]
- Stein C. Peripheral analgesic actions of opioids. J Pain Symptom Manage. 1991 Apr;6(3):119–124. doi: 10.1016/0885-3924(91)90960-c. [DOI] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995 Jun 22;332(25):1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Eisenstein T. K., Geller E. B., Adler M. W., Rogers T. J. Immunomodulatory activity of mu- and kappa-selective opioid agonists. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. S., Howlett C. R., Nayanar V. Anti-inflammatory effects of kappa-opioids in adjuvant arthritis. Life Sci. 1995;57(4):371–378. doi: 10.1016/0024-3205(95)00296-i. [DOI] [PubMed] [Google Scholar]
- Walker J. S., Kasmerski L. Diflunisal pharmacodynamics in experimental arthritis in rats. J Rheumatol. 1988 Nov;15(11):1643–1647. [PubMed] [Google Scholar]
- Watkins L. R., Maier S. F., Goehler L. E. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995 Dec;63(3):289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Wilske K. R., Healey L. A. Remodeling the pyramid--a concept whose time has come. J Rheumatol. 1989 May;16(5):565–567. [PubMed] [Google Scholar]
- Yaksh T. L. Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res. 1988 Aug 23;458(2):319–324. doi: 10.1016/0006-8993(88)90474-x. [DOI] [PubMed] [Google Scholar]