Abstract
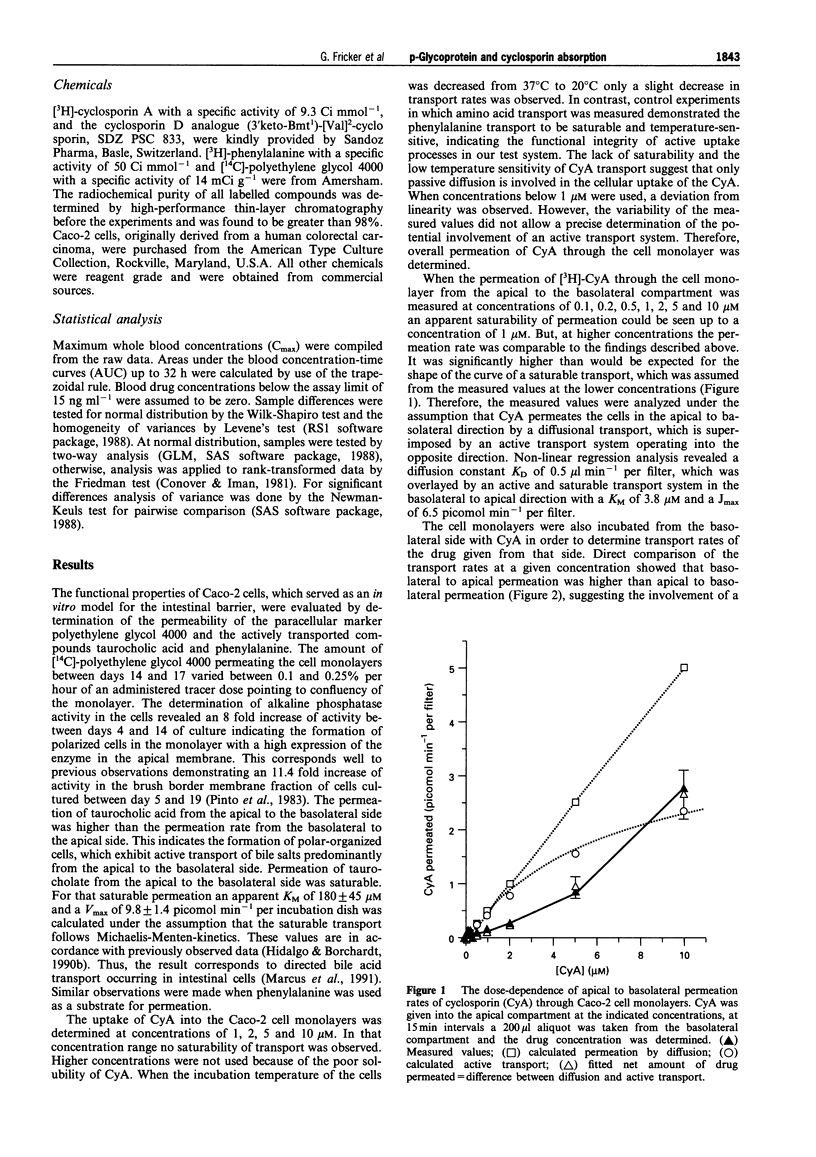
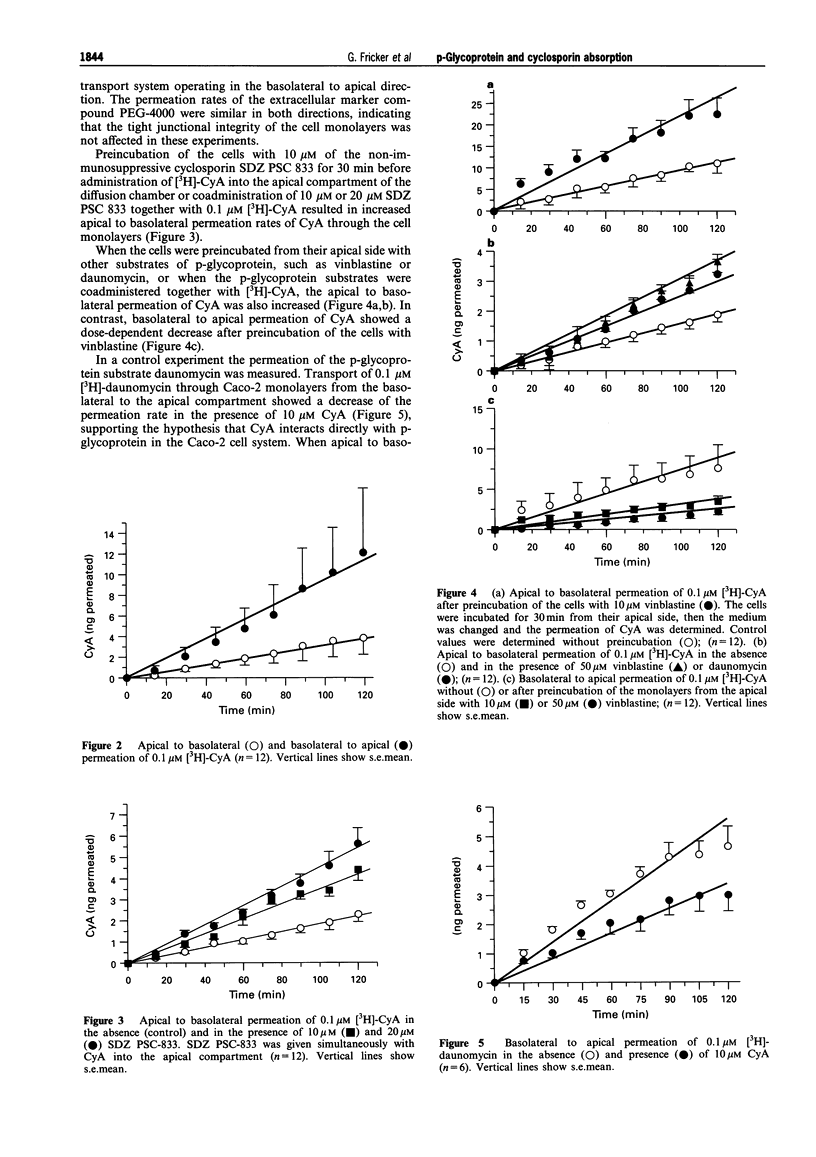
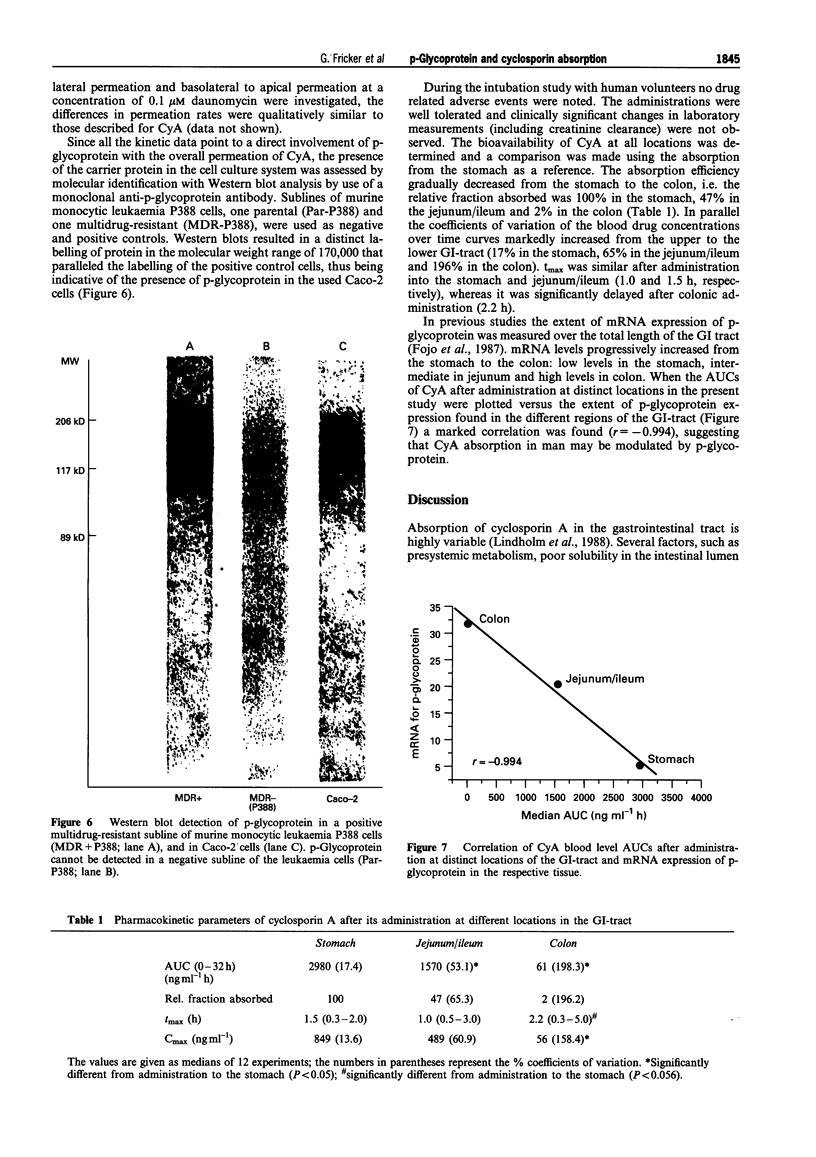
1. The interaction of cyclosporin A (CyA) with p-glycoprotein during intestinal uptake was investigated by a combination of in vitro experiments with human Caco-2 cells and an intubation study in healthy volunteers. 2. CyA uptake into the cells was not saturable and exhibited only a low temperature sensitivity, suggesting passive diffusion. When the permeation of CyA across Caco-2 monolayers from the apical to the basolateral side was determined, overall transport had an apparently saturable component up to a concentration of 1 microM. At higher concentrations permeation increased over-proportionally. Calculation of the kinetic parameters of apical to basolateral permeation suggested a diffusional process with a KD of 0.5 microliter min-1 per filter, which was overlayed by an active system in basolateral to apical direction with a KM of 3.8 microM and a Jmax of 6.5 picomol min-1 per filter. 3. CyA permeation was significantly higher when the drug was given from the basolateral side as compared to the permeation from the apical side. Apical to basolateral transport of CyA was increased in the presence of vinblastine, daunomycin and a non-immunosuppressive CyA-derivative. All compounds inhibit p-glycoprotein-mediated transport processes. Basolateral to apical permeation of CyA showed a dose-dependent decrease in the presence of vinblastine. Permeation of daunomycin across Caco-2 cell monolayers was also higher from the basolateral to the apical side than vice versa. Basolateral to apical permeation was decreased in the presence of SDZ PSC 833 and cyclosporin A. 4. Western blot analysis of Caco-2 cells with the monoclonal antibody C219 confirmed the presence of p-glycoprotein in the used cell system. 5. When the absorption of CyA in the gastrointestinal (GI)-tract of healthy volunteers was determined, a remarkable decrease of the plasma AUC could be observed dependent on the location of absorption in the rank order stomach > jejunum/ileum > colon. The decrease in absorption exhibited a marked correlation (r = 0.994) to the expression of mRNA for p-glycoprotein over the GI-tract (stomach < jejunum < colon). 6. All data provide evidence that CyA is a substrate of p-glycoprotein in the GI-tract, which might explain the local differences and the high variability in cyclosporin absorption found in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audus K. L., Bartel R. L., Hidalgo I. J., Borchardt R. T. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm Res. 1990 May;7(5):435–451. doi: 10.1023/a:1015800312910. [DOI] [PubMed] [Google Scholar]
- Ball P. E., Munzer H., Keller H. P., Abisch E., Rosenthaler J. Specific 3H radioimmunoassay with a monoclonal antibody for monitoring cyclosporine in blood. Clin Chem. 1988 Feb;34(2):257–260. [PubMed] [Google Scholar]
- Boesch D., Gavériaux C., Jachez B., Pourtier-Manzanedo A., Bollinger P., Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991 Aug 15;51(16):4226–4233. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Pentlow B. D., Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978 Dec 23;2(8104-5):1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo I. J., Borchardt R. T. Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta. 1990 Sep 21;1028(1):25–30. doi: 10.1016/0005-2736(90)90261-l. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Borchardt R. T. Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta. 1990 Jul 20;1035(1):97–103. doi: 10.1016/0304-4165(90)90179-z. [DOI] [PubMed] [Google Scholar]
- Hunter J., Jepson M. A., Tsuruo T., Simmons N. L., Hirst B. H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J Biol Chem. 1993 Jul 15;268(20):14991–14997. [PubMed] [Google Scholar]
- Ito S., Koren G., Harper P. A., Silverman M. Energy-dependent transport of digoxin across renal tubular cell monolayers (LLC-PK1). Can J Physiol Pharmacol. 1993 Jan;71(1):40–47. doi: 10.1139/y93-006. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Kuo S. M., Ziemniak J., Artursson P. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. Br J Pharmacol. 1993 Nov;110(3):1009–1016. doi: 10.1111/j.1476-5381.1993.tb13914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefee E. B., Scharschmidt B. F., Blankenship N. M., Ockner R. K. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979 Dec;64(6):1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg-Freijs A., Karlsson M. O. Dose dependent absorption and linear disposition of cyclosporin A in rat. Biopharm Drug Dispos. 1994 Jan;15(1):75–86. doi: 10.1002/bdd.2510150107. [DOI] [PubMed] [Google Scholar]
- Lindholm A., Henricsson S., Lind M., Dahlqvist R. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol. 1988;34(5):461–464. doi: 10.1007/BF01046702. [DOI] [PubMed] [Google Scholar]
- Lum B. L., Fisher G. A., Brophy N. A., Yahanda A. M., Adler K. M., Kaubisch S., Halsey J., Sikic B. I. Clinical trials of modulation of multidrug resistance. Pharmacokinetic and pharmacodynamic considerations. Cancer. 1993 Dec 1;72(11 Suppl):3502–3514. doi: 10.1002/1097-0142(19931201)72:11+<3502::aid-cncr2820721618>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Marcus S. N., Schteingart C. D., Marquez M. L., Hofmann A. F., Xia Y., Steinbach J. H., Ton-Nu H. T., Lillienau J., Angellotti M. A., Schmassmann A. Active absorption of conjugated bile acids in vivo. Kinetic parameters and molecular specificity of the ileal transport system in the rat. Gastroenterology. 1991 Jan;100(1):212–221. doi: 10.1016/0016-5085(91)90603-i. [DOI] [PubMed] [Google Scholar]
- Mehta M. U., Venkataramanan R., Burckart G. J., Ptachcinski R. J., Delamos B., Stachak S., Van Thiel D. H., Iwatsuki S., Starzl T. E. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988 May;25(5):579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptachcinski R. J., Burckart G. J., Venkataramanan R. Cyclosporine. Drug Intell Clin Pharm. 1985 Feb;19(2):90–100. doi: 10.1177/106002808501900202. [DOI] [PubMed] [Google Scholar]
- Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993 Mar 25;268(9):6077–6080. [PubMed] [Google Scholar]
- Sakata A., Tamai I., Kawazu K., Deguchi Y., Ohnishi T., Saheki A., Tsuji A. In vivo evidence for ATP-dependent and P-glycoprotein-mediated transport of cyclosporin A at the blood-brain barrier. Biochem Pharmacol. 1994 Nov 16;48(10):1989–1992. doi: 10.1016/0006-2952(94)90601-7. [DOI] [PubMed] [Google Scholar]
- Schramm U., Fricker G., Wenger R., Miller D. S. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol. 1995 Jan;268(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Speeg K. V., Maldonado A. L. Effect of the nonimmunosuppressive cyclosporin analog SDZ PSC-833 on colchicine and doxorubicin biliary secretion by the rat in vivo. Cancer Chemother Pharmacol. 1994;34(2):133–136. doi: 10.1007/BF00685930. [DOI] [PubMed] [Google Scholar]
- Takeguchi N., Ichimura K., Koike M., Matsui W., Kashiwagura T., Kawahara K. Inhibition of the multidrug efflux pump in isolated hepatocyte couplets by immunosuppressants FK506 and cyclosporine. Transplantation. 1993 Mar;55(3):646–650. doi: 10.1097/00007890-199303000-00033. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda C. T., Lemaire M., Gsell G., Misslin P., Nussbaumer K. Apparent dose-dependent oral absorption of cyclosporin A in rats. Biopharm Drug Dispos. 1984 Apr-Jun;5(2):141–151. doi: 10.1002/bdd.2510050207. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R., Burckhart G. J., Ptachcinski R. J. Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Semin Liver Dis. 1985 Nov;5(4):357–368. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]
- Vickers A. E., Fischer V., Connors S., Fisher R. L., Baldeck J. P., Maurer G., Brendel K. Cyclosporin A metabolism in human liver, kidney, and intestine slices. Comparison to rat and dog slices and human cell lines. Drug Metab Dispos. 1992 Nov-Dec;20(6):802–809. [PubMed] [Google Scholar]
- Wassef R., Cohen Z., Langer B. Pharmacokinetic profiles of cyclosporine in rats. Influence of route of administration and dosage. Transplantation. 1985 Nov;40(5):489–493. doi: 10.1097/00007890-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Webber I. R., Peters W. H., Back D. J. Cyclosporin metabolism by human gastrointestinal mucosal microsomes. Br J Clin Pharmacol. 1992 Jun;33(6):661–664. doi: 10.1111/j.1365-2125.1992.tb04098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacherl J., Hamilton G., Thalhammer T., Riegler M., Cosentini E. P., Ellinger A., Bischof G., Schweitzer M., Teleky B., Koperna T. Inhibition of P-glycoprotein-mediated vinblastine transport across HCT-8 intestinal carcinoma monolayers by verapamil, cyclosporine A and SDZ PSC 833 in dependence on extracellular pH. Cancer Chemother Pharmacol. 1994;34(2):125–132. doi: 10.1007/BF00685929. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M., Koepsell H. Photoaffinity labeling of membrane proteins from rat liver and pig kidney with cyclosporine diazirine. Involvement of binding to plasma membranes cytotoxic effects. Transplantation. 1988 Aug;46(2 Suppl):15S–20S. [PubMed] [Google Scholar]
- van Kalken C. K., Broxterman H. J., Pinedo H. M., Feller N., Dekker H., Lankelma J., Giaccone G. Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer. 1993 Feb;67(2):284–289. doi: 10.1038/bjc.1993.54. [DOI] [PMC free article] [PubMed] [Google Scholar]


