Abstract
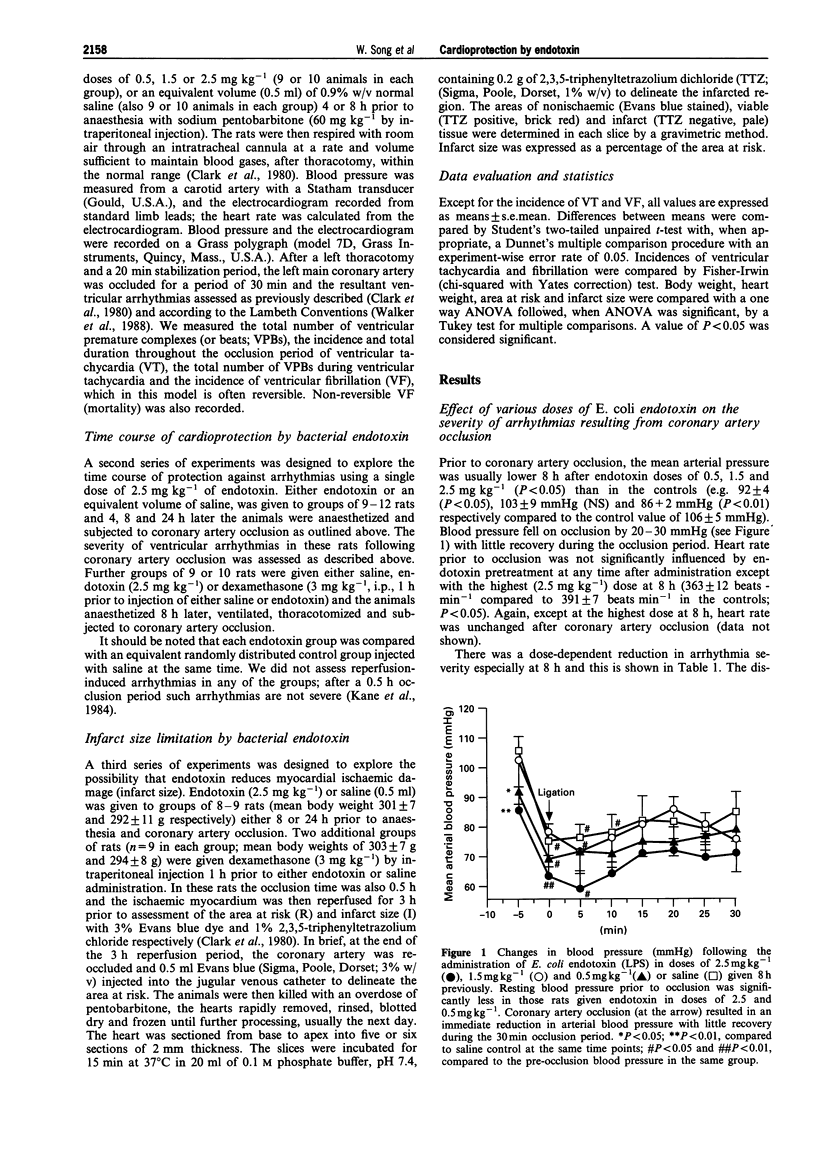
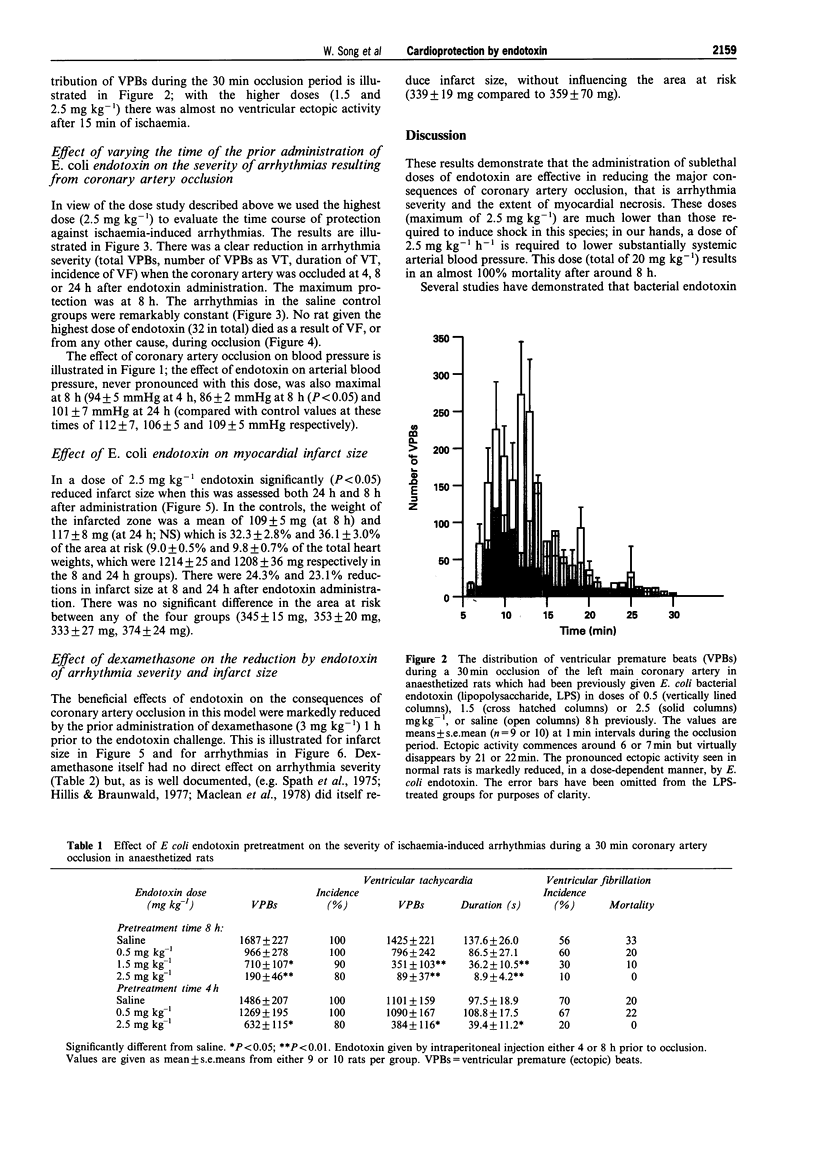
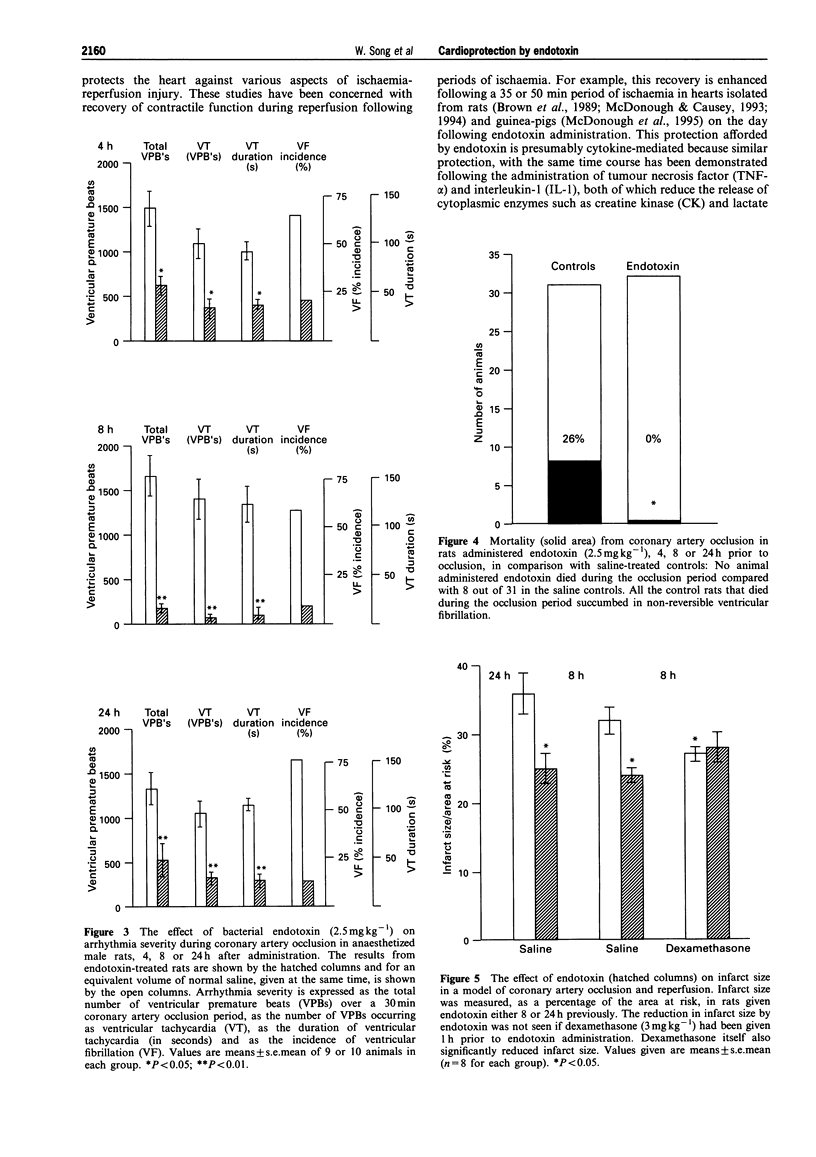
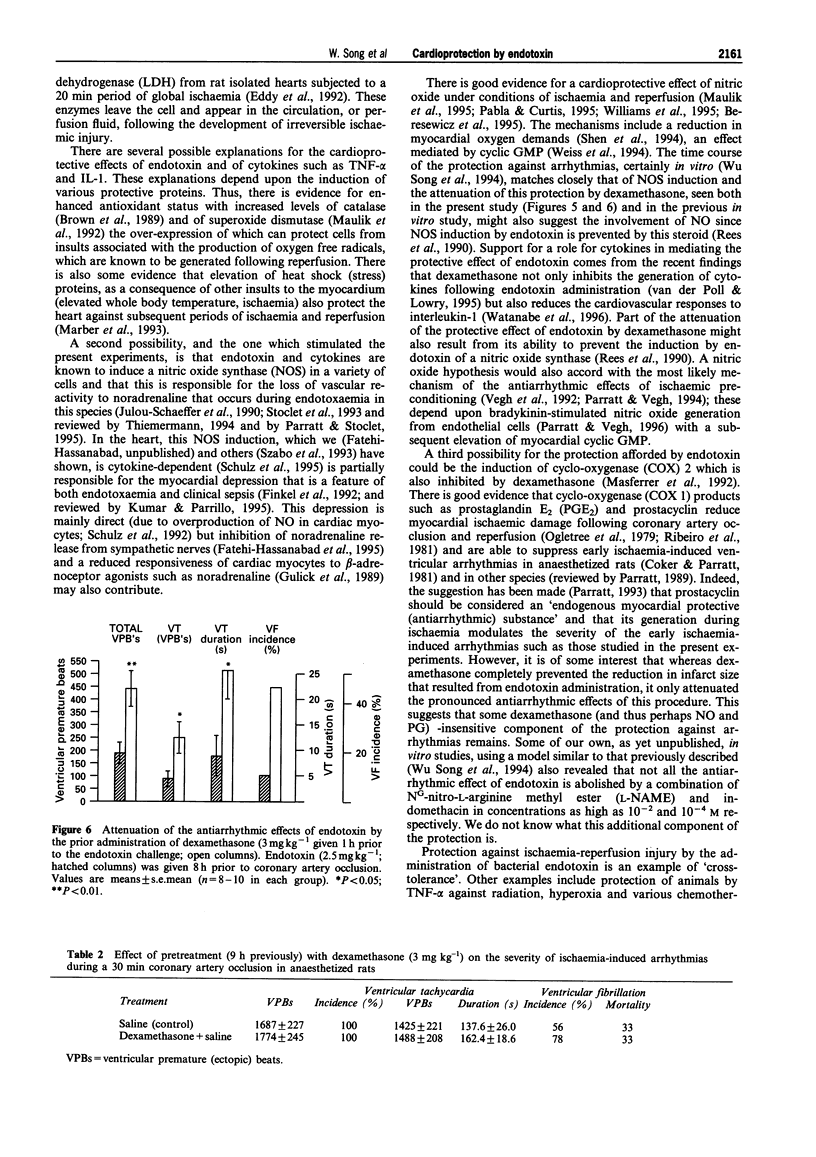
1. Bacterial endotoxin (lipopolysaccharide derived from Escherichia coli) was injected intraperitoneally in conscious rats in doses ranging from 0.5 to 2.5 mg kg-1. At various times afterwards the animals were anaesthetized and subjected to a 30 min period of left coronary artery occlusion. 2. Under these conditions the severity of ventricular arrhythmias was markedly suppressed, in comparison with saline-injected controls, but this was particularly marked with the higher doses (1.5 and 2.5 mg kg-1); the number of ventricular premature beats was reduced from 1687 +/- 227 over the 0.5 h coronary artery occlusion period to 190 +/- 46 in those rats administered 2.5 mg kg-1 endotoxin 8 h previously (P < 0.05). The duration of ventricular tachycardia was also significantly reduced (138 +/- 26 s to 8.9 +/- 4.2 s; P < 0.01) and there was a reduction in the incidence of ventricular fibrillation (from 56% to 10%). 3. The time course of this protection was studied following the administration of a single dose of 2.5 mg kg-1 of endotoxin by anaesthetizing rats 4, 8 or 24 h later. Protection was apparent at each time but was particularly marked at 8 h. 4. No rat given the highest dose of endotoxin (32 in all) died as a result of ventricular fibrillation, or from any other cause, during an occlusion, in contrast to a 26% mortality in the controls (P < 0.01). 5. Infarct size, measured following a 30 min period of coronary artery occlusion followed by a 3 h reperfusion period, was reduced both 8 and 24 h after the administration of 2.5 mg kg-1 endotoxin (reductions of 24.3 and 23.1% respectively; P < 0.05). Endotoxin had no significant effect on the area at risk. 6. The beneficial effects of endotoxin on infarct size and on ventricular arrhythmias were markedly attenuated by the prior administration of dexamethasone, 3 mg kg-1 given 1 h prior to endotoxin administration. Dexamethasone itself reduced infarct size (P < 0.05) but had no direct effect on arrhythmia severity following coronary artery occlusion. 7. The mechanisms of this "cross-tolerance' induced by bacterial endotoxin against ischaemia-reperfusion injury remain to be elucidated but the most likely mechanisms appear to be the induction of protective enzymes or proteins (e.g. nitric oxide synthase, cyclo-oxygenase (COX) 2) probably mediated by cytokine release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beresewicz A., Karwatowska-Prokopczuk E., Lewartowski B., Cedro-Ceremuåzyńska K. A protective role of nitric oxide in isolated ischaemic/reperfused rat heart. Cardiovasc Res. 1995 Dec;30(6):1001–1008. doi: 10.1016/s0008-6363(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Grosso M. A., Terada L. S., Whitman G. J., Banerjee A., White C. W., Harken A. H., Repine J. E. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Foreman M. I., Kane K. A., McDonald F. M., Parratt J. R. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J Pharmacol Methods. 1980 Jun;3(4):357–368. doi: 10.1016/0160-5402(80)90077-7. [DOI] [PubMed] [Google Scholar]
- Coker S. J., Parratt J. R. The effects of prostaglandins E2, F2 alpha, prostacyclin, flurbiprofen and aspirin on arrhythmias resulting from coronary artery ligation in anaesthetized rats. Br J Pharmacol. 1981 Sep;74(1):155–159. doi: 10.1111/j.1476-5381.1981.tb09968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy L. J., Goeddel D. V., Wong G. H. Tumor necrosis factor-alpha pretreatment is protective in a rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1056–1059. doi: 10.1016/0006-291x(92)90698-k. [DOI] [PubMed] [Google Scholar]
- Eising G. P., Mao L., Schmid-Schonbein G. W., Engler R. L., Ross J. Effects of induced tolerance to bacterial lipopolysaccharide on myocardial infarct size in rats. Cardiovasc Res. 1996 Jan;31(1):73–81. [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z., Furman B. L., Parratt J. R. The effect of endotoxin on sympathetic responses in the rat isolated perfused mesenteric bed; involvement of nitric oxide and cyclo-oxygenase products. Br J Pharmacol. 1995 Dec;116(8):3316–3322. doi: 10.1111/j.1476-5381.1995.tb15141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis L. D., Braunwald E. Myocardial ischemia (third of three parts). N Engl J Med. 1977 May 12;296(19):1093–1096. doi: 10.1056/NEJM197705122961905. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kane K. A., Parratt J. R., Williams F. M. An investigation into the characteristics of reperfusion-induced arrhythmias in the anaesthetized rat and their susceptibility to antiarrhythmic agents. Br J Pharmacol. 1984 Jun;82(2):349–357. doi: 10.1111/j.1476-5381.1984.tb10769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya T., Hoshida S., Yamashita N., Fuji H., Oe H., Hori M., Kamada T., Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993 Jun;72(6):1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Maclean D., Fishbein M. C., Braunwald E., Maroko P. R. Long-term preservation of ischemic myocardium after experimental coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):541–551. doi: 10.1172/JCI108965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber M. S., Latchman D. S., Walker J. M., Yellon D. M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993 Sep;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Walker J. M., Latchman D. S., Yellon D. M. Myocardial protection after whole body heat stress in the rabbit is dependent on metabolic substrate and is related to the amount of the inducible 70-kD heat stress protein. J Clin Invest. 1994 Mar;93(3):1087–1094. doi: 10.1172/JCI117059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik N., Engelman D. T., Watanabe M., Engelman R. M., Maulik G., Cordis G. A., Das D. K. Nitric oxide signaling in ischemic heart. Cardiovasc Res. 1995 Oct;30(4):593–601. [PubMed] [Google Scholar]
- McDonough K. H., Causey K. M. Effects of sepsis on recovery of the heart from 50 min ischemia. Shock. 1994 Jun;1(6):432–437. doi: 10.1097/00024382-199406000-00007. [DOI] [PubMed] [Google Scholar]
- McDonough K. H., Giaimo M. E., Miller H. I. Effects of endotoxin on the guinea pig heart response to ischemia reperfusion injury. Shock. 1995 Aug;4(2):139–142. doi: 10.1097/00024382-199508000-00010. [DOI] [PubMed] [Google Scholar]
- Nelson D. W., Brown J. M., Banerjee A., Bensard D. D., Rogers K. B., Locke-Winter C. R., Anderson B. O., Harken A. H. Pretreatment with a nontoxic derivative of endotoxin induces functional protection against cardiac ischemia/reperfusion injury. Surgery. 1991 Aug;110(2):365–369. [PubMed] [Google Scholar]
- Ogletree M. L., Lefer A. M., Smith J. B., Nicolaou K. C. Studies on the protective effect of prostacyclin in acute myocardial ischemia. Eur J Pharmacol. 1979 Jun;56(1-2):95–103. doi: 10.1016/0014-2999(79)90438-2. [DOI] [PubMed] [Google Scholar]
- Pabla R., Curtis M. J. Effects of NO modulation on cardiac arrhythmias in the rat isolated heart. Circ Res. 1995 Nov;77(5):984–992. doi: 10.1161/01.res.77.5.984. [DOI] [PubMed] [Google Scholar]
- Parratt J. R., Szekeres L. Delayed protection of the heart against ischaemia. Trends Pharmacol Sci. 1995 Oct;16(10):351–355. doi: 10.1016/s0165-6147(00)89069-0. [DOI] [PubMed] [Google Scholar]
- Parratt J. R., Vegh A. Endothelial cells, nitric oxide and ischaemic preconditioning. Basic Res Cardiol. 1996 Jan-Feb;91(1):27–30. doi: 10.1007/BF00788857. [DOI] [PubMed] [Google Scholar]
- Parratt J. Endogenous myocardial protective (antiarrhythmic) substances. Cardiovasc Res. 1993 May;27(5):693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Parratt J., Vegh A. Pronounced antiarrhythmic effects of ischemic preconditioning. Cardioscience. 1994 Mar;5(1):9–18. [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. Preconditioning: a balanced perspective. Br Heart J. 1995 Dec;74(6):575–577. doi: 10.1136/hrt.74.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro L. G., Brandon T. A., Hopkins D. G., Reduto L. A., Taylor A. A., Miller R. R. Prostacyclin in experimental myocardial ischemia: effects on hemodynamics, regional myocardial blood flow, infarct size and mortality. Am J Cardiol. 1981 Apr;47(4):835–840. doi: 10.1016/0002-9149(81)90182-x. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Panas D. L., Catena R., Moncada S., Olley P. M., Lopaschuk G. D. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br J Pharmacol. 1995 Jan;114(1):27–34. doi: 10.1111/j.1476-5381.1995.tb14901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Xu X., Ochoa M., Zhao G., Wolin M. S., Hintze T. H. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994 Dec;75(6):1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Song W., Furman B. L., Parratt J. R. Attenuation by dexamethasone of endotoxin protection against ischaemia-induced ventricular arrhythmias. Br J Pharmacol. 1994 Dec;113(4):1083–1084. doi: 10.1111/j.1476-5381.1994.tb17105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath J. A., Lefer A. M. Effects of dexamethasone on myocardial cells in the early phase of acute myocardial infarction. Am Heart J. 1975 Jul;90(1):50–55. doi: 10.1016/0002-8703(75)90256-2. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres L., Papp J. G., Szilvássy Z., Udvary E., Vegh A. Moderate stress by cardiac pacing may induce both short term and long term cardioprotection. Cardiovasc Res. 1993 Apr;27(4):593–596. doi: 10.1093/cvr/27.4.593. [DOI] [PubMed] [Google Scholar]
- Szekeres L., Szilvássy Z., Udvary E., Végh A. 7-oxo-PgI2 induced late appearing and long-lasting electrophysiological changes in the heart in situ of the rabbit, guinea-pig, dog and cat. J Mol Cell Cardiol. 1989 Jun;21(6):545–554. doi: 10.1016/0022-2828(89)90820-1. [DOI] [PubMed] [Google Scholar]
- Thiemermann C. The role of the L-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- Vegh A., Papp J. G., Parratt J. R. Prevention by dexamethasone of the marked antiarrhythmic effects of preconditioning induced 20 h after rapid cardiac pacing. Br J Pharmacol. 1994 Dec;113(4):1081–1082. doi: 10.1111/j.1476-5381.1994.tb17104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegh A., Szekeres L., Parratt J. Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol. 1992 Nov;107(3):648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. J., Curtis M. J., Hearse D. J., Campbell R. W., Janse M. J., Yellon D. M., Cobbe S. M., Coker S. J., Harness J. B., Harron D. W. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988 Jul;22(7):447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tan N., Saiki Y., Makisumi T., Nakamura S. Possible involvement of glucocorticoids in the modulation of interleukin-1-induced cardiovascular responses in rats. J Physiol. 1996 Feb 15;491(Pt 1):231–239. doi: 10.1113/jphysiol.1996.sp021211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. R., Rodriguez E., Tse J., Scholz P. M. Effect of increased myocardial cyclic GMP induced by cyclic GMP-phosphodiesterase inhibition on oxygen consumption and supply of rabbit hearts. Clin Exp Pharmacol Physiol. 1994 Aug;21(8):607–614. doi: 10.1111/j.1440-1681.1994.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Williams M. W., Taft C. S., Ramnauth S., Zhao Z. Q., Vinten-Johansen J. Endogenous nitric oxide (NO) protects against ischaemia-reperfusion injury in the rabbit. Cardiovasc Res. 1995 Jul;30(1):79–86. [PubMed] [Google Scholar]
- Yao Z., Auchampach J. A., Pieper G. M., Gross G. J. Cardioprotective effects of monophosphoryl lipid A, a novel endotoxin analogue, in the dog. Cardiovasc Res. 1993 May;27(5):832–838. doi: 10.1093/cvr/27.5.832. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Baxter G. F. A "second window of protection" or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol. 1995 Apr;27(4):1023–1034. doi: 10.1016/0022-2828(95)90071-3. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Lowry S. F. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995 Jan;3(1):1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]


