Abstract
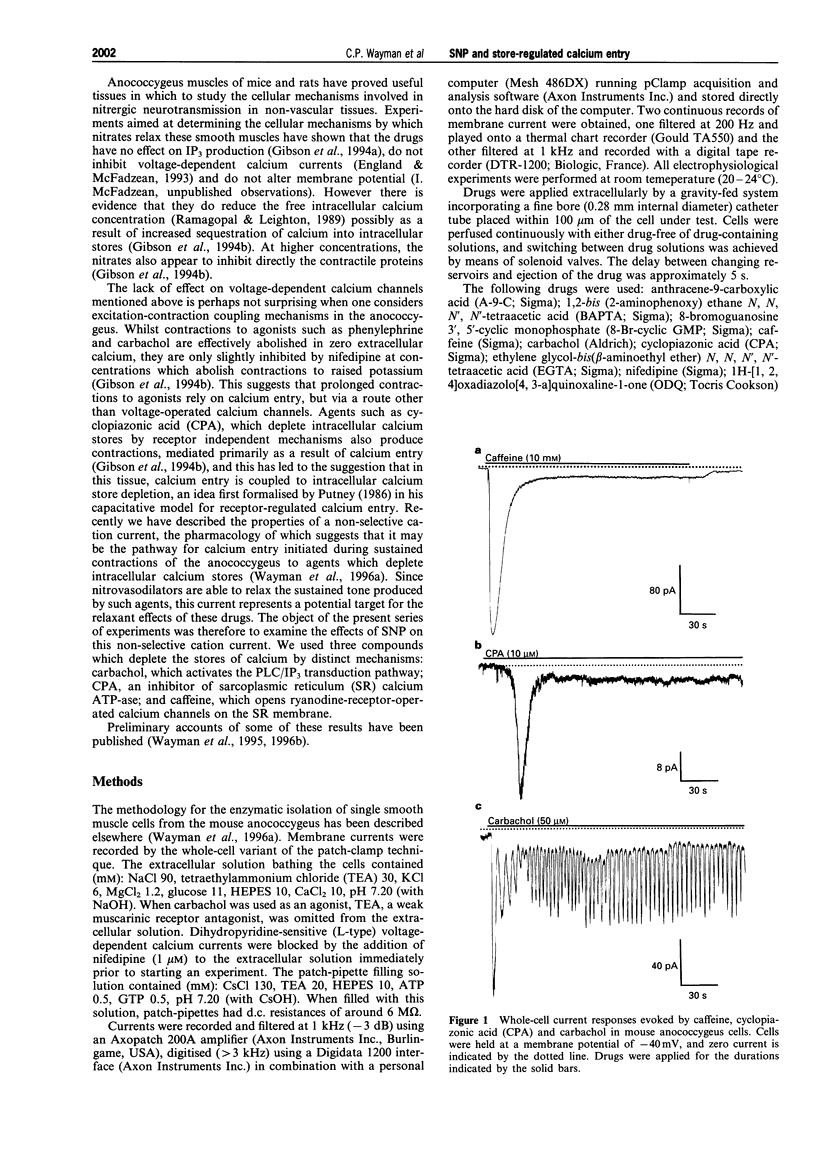
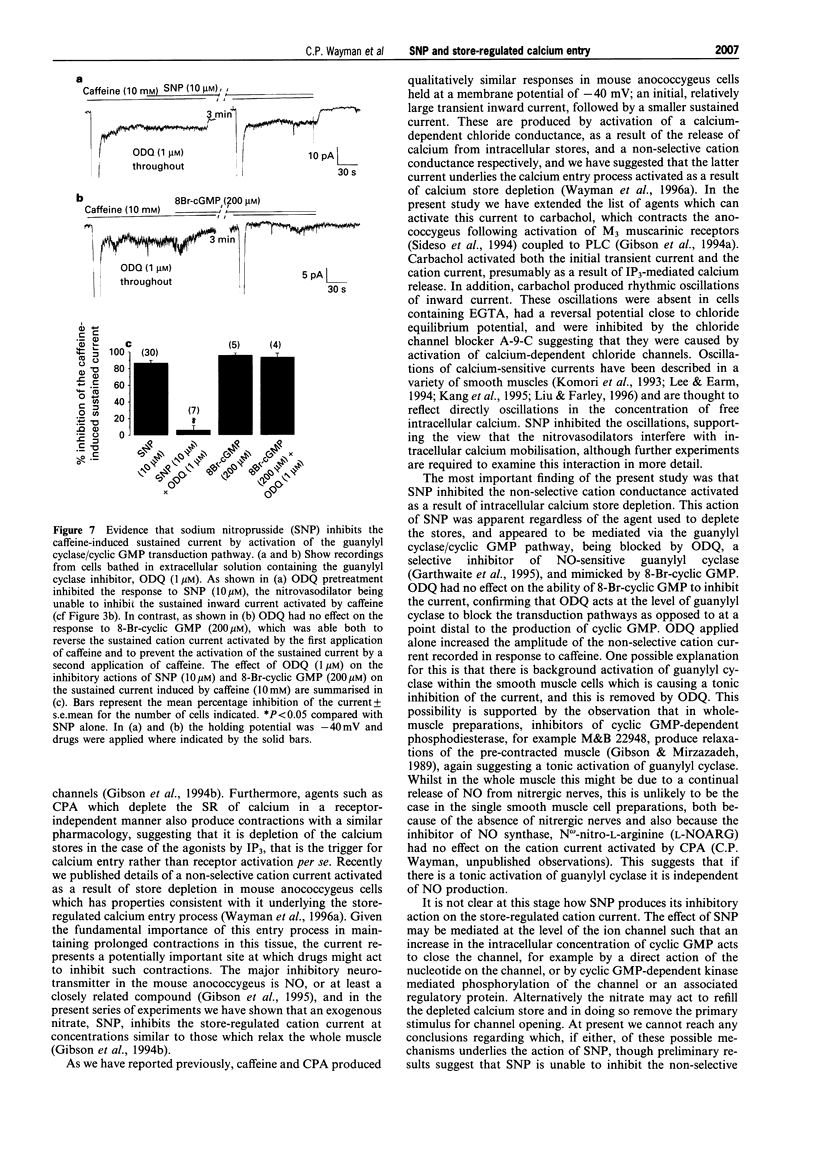
1. The effects of sodium nitroprusside (SNP) on the non-selective cation current activated in response to intracellular calcium store depletion were studied using the whole-cell patch-clamp technique in single smooth muscle cells isolated from the mouse anococcygeus. Voltage-dependent calcium currents were blocked with extracellular nifedipine, and caesium and tetraethylammonium chloride were used to block voltage-dependent potassium currents. Calcium stores were depleted with caffeine (10 mM), carbachol (50 microM) or cyclopiazonic acid (CPA 10 microM; an inhibitor of the sarcoplasmic reticulum [SR] calcium-ATPase). 2. At a holding potential of -40 mV, both CPA and caffeine activated inward currents which consisted of two clearly distinguishable components; an initial transient current followed by a smaller sustained current. In the case of CPA, the amplitudes of the transient and sustained components were 19.7 +/- 2.1 pA and 3.5 +/- 0.3 pA respectively, whilst the equivalent values for caffeine were 188 +/- 21 and 4.8 +/- 0.3 pA. As described previously, the transient current results from activation of a calcium-dependent chloride conductance whilst the sustained current is a non-selective cation current, activated following intracellular calcium store depletion. 3. The muscarinic receptor agonist, carbachol, also activated a transient followed by a sustained current with amplitudes of 238 +/- 55 and 4.7 +/- 0.5 pA respectively. Superimposed on the sustained current were regular, oscillations of calcium-activated chloride current. 4. Both the transient and the sustained currents activated by CPA were absent in cells pretreated with SNP (10 microM). Application of SNP to a cell following activation of the sustained current by CPA inhibited the current by 88.6 +/- 3.8%. SNP (10 microM) did not inhibit the transient current activated by caffeine but abolished the sustained current. 5. SNP (10 microM) had no effect on the initial transient current activated by carbachol (50 microM). However, it did inhibit the oscillations in the inward current. In recordings from cells bathed in extracellular solution containing the chloride channel blocker, anthracene-9-carboxylic acid (A-9-C; 1 mM), carbachol activated only a sustained current. This current was inhibited by 88.1 +/- 6.5% by a concomitant application of SNP (10 microM) and was absent in cells pretreated with the nitrovasodilator. 6. The effects of SNP on the currents activated by caffeine (10 mM) were mimicked by 8-bromo-cyclic GMP (200 microM); thus the nucleotide had no effect on the transient current activated by caffeine but abolished the sustained current. The effects of SNP, but not those of 8-bromo-cyclic GMP, were inhibited by the nitric oxide-sensitive guanylyl cyclase inhibitor, 1H-[1, 2, 4]oxadiazolo[4, 3-a]quinoxaline-1-one (ODQ; 1 microM). ODQ alone produced a significant increase in the size of the sustained current activated by caffeine (7.8 +/- 0.7 pA). 7. These findings suggest that SNP activates guanylyl cyclase to inhibit the non-selective cation current activated as a result of intracellular calcium store depletion in mouse anococcygeus cells. Since the non-selective cation current appears to underlie the calcium entry process responsible for maintaining the sustained contractions to agonists in this tissue, this action of SNP may represent an important mechanism by which nitrates relax non-vascular smooth muscle.
Full text
PDF

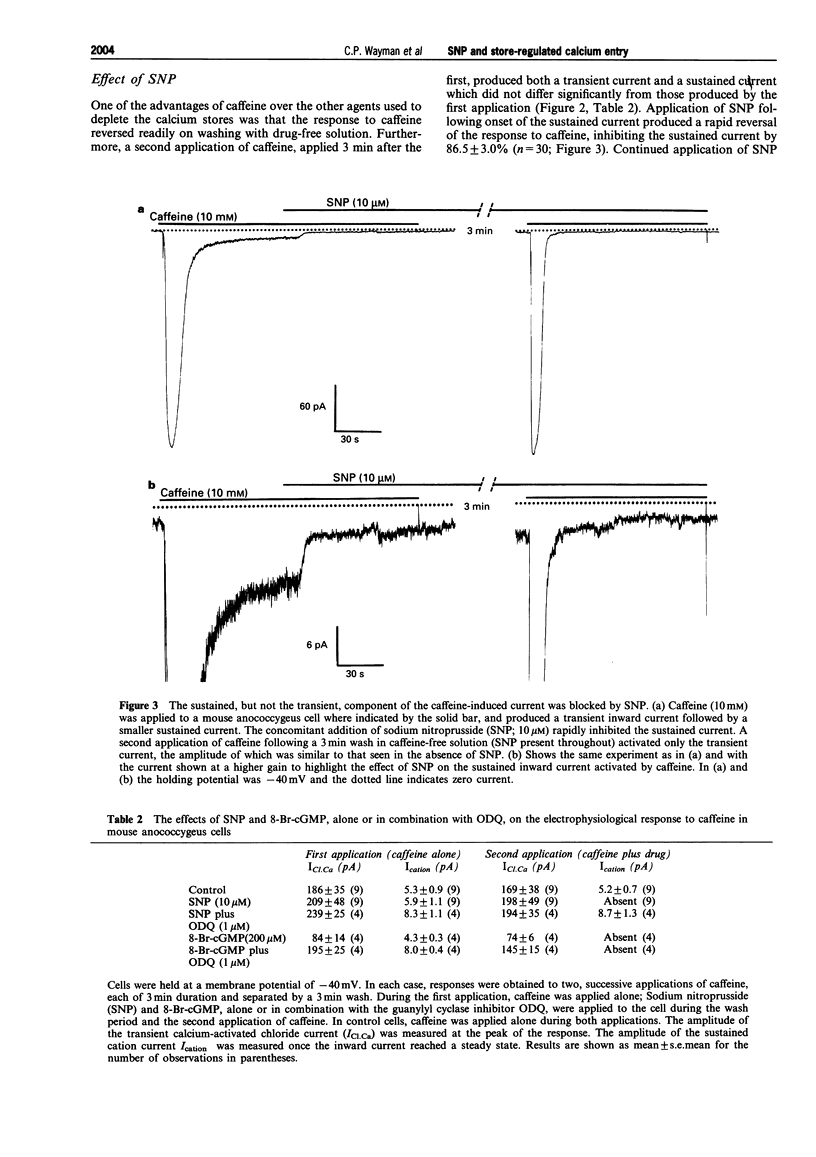
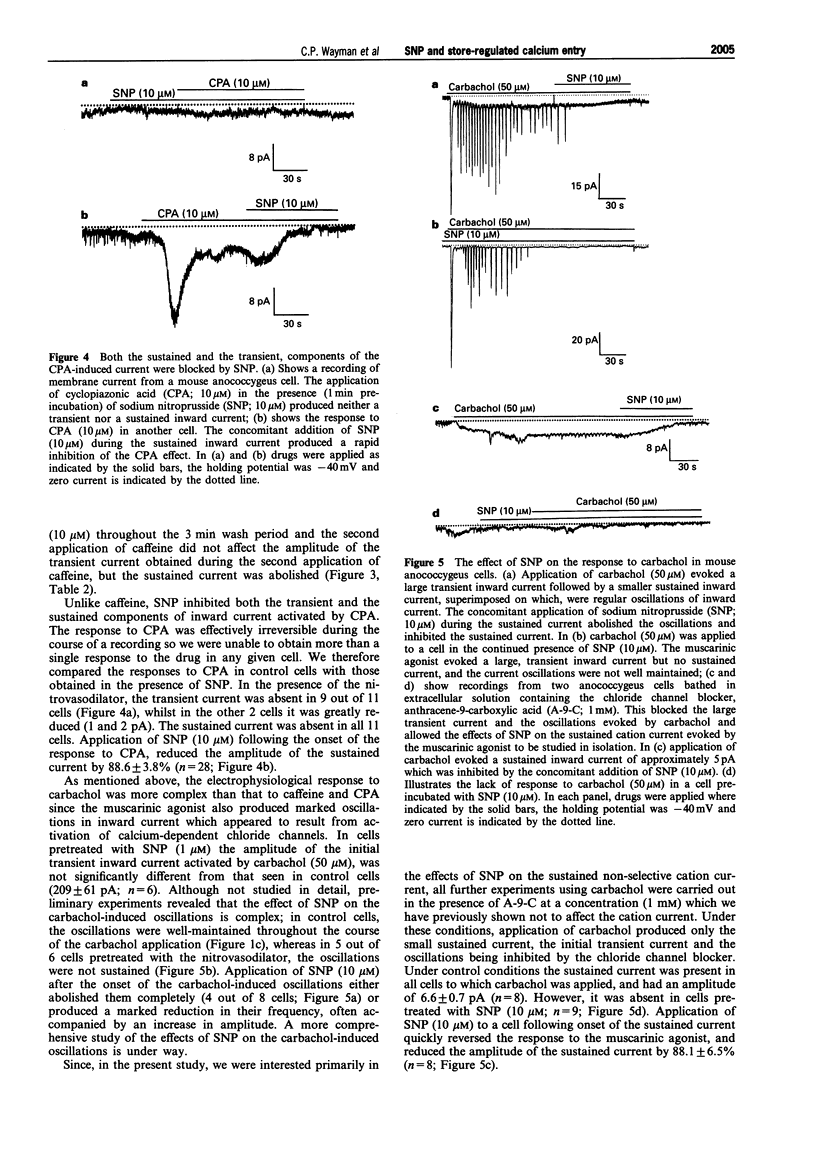

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
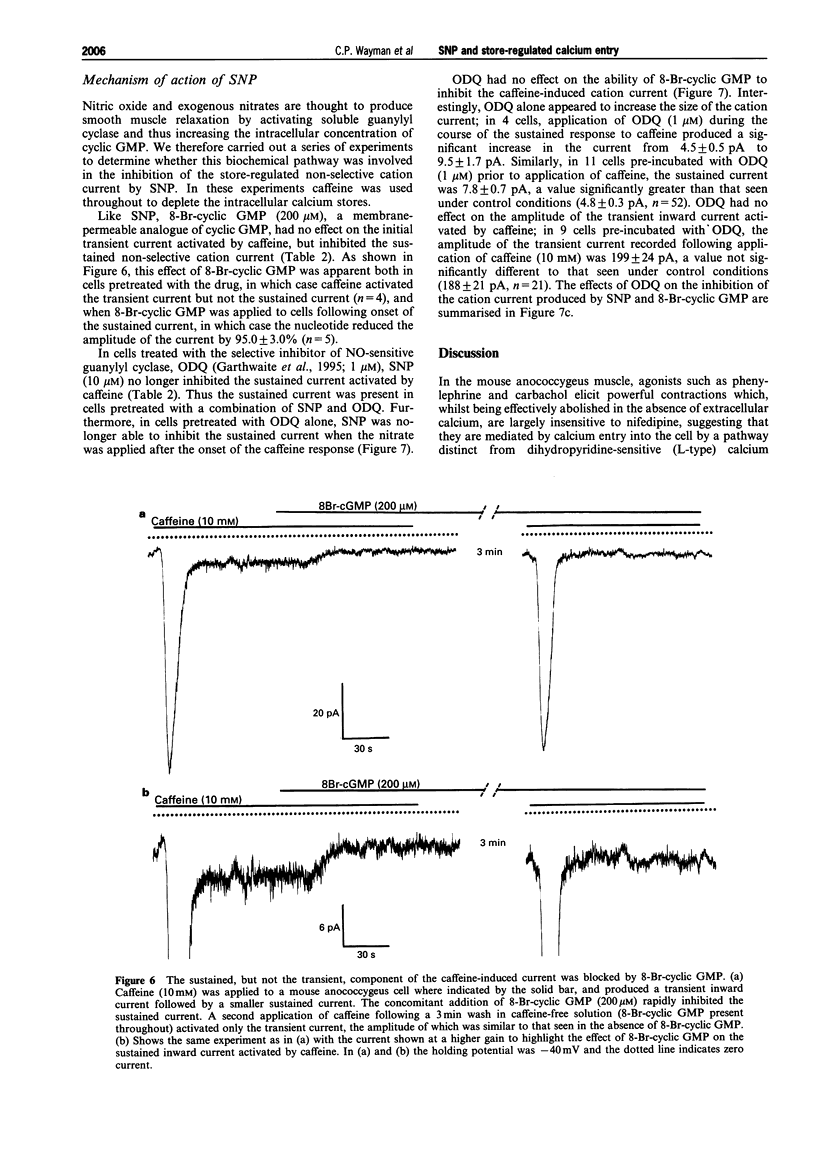
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Pryzwansky K. B., Wyatt T. A., Lincoln T. M. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991 Dec;40(6):923–931. [PubMed] [Google Scholar]
- Garthwaite J., Southam E., Boulton C. L., Nielsen E. B., Schmidt K., Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995 Aug;48(2):184–188. [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Mirzazadeh S., Tucker J. F., Wayman C. Nitrergic stimulation does not inhibit carbachol-induced inositol phosphate generation in the rat anococcygeus. Neurosci Lett. 1994 Aug 29;178(1):35–38. doi: 10.1016/0304-3940(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Tucker J. F., Wayman C. The nitrergic transmitter of the anococcygeus--NO or not? Arch Int Pharmacodyn Ther. 1995 Jan-Feb;329(1):39–51. [PubMed] [Google Scholar]
- Gibson A., McFadzean I., Tucker J. F., Wayman C. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br J Pharmacol. 1994 Dec;113(4):1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Suzuki M., Uyama Y., Muraki K., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses and an outward current in smooth muscle. Jpn J Pharmacol. 1992;58 (Suppl 2):401P–401P. [PubMed] [Google Scholar]
- Kang T. M., So I., Kim K. W. Caffeine- and histamine-induced oscillations of K(Ca) current in single smooth muscle cells of rabbit cerebral artery. Pflugers Arch. 1995 Nov;431(1):91–100. doi: 10.1007/BF00374381. [DOI] [PubMed] [Google Scholar]
- Komori S., Kawai M., Pacaud P., Ohashi H., Bolton T. B. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflugers Arch. 1993 Sep;424(5-6):431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Earm Y. E. Caffeine induces periodic oscillations of Ca(2+)-activated K+ current in pulmonary arterial smooth muscle cells. Pflugers Arch. 1994 Feb;426(3-4):189–198. doi: 10.1007/BF00374771. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M. Cyclic GMP and mechanisms of vasodilation. Pharmacol Ther. 1989;41(3):479–502. doi: 10.1016/0163-7258(89)90127-7. [DOI] [PubMed] [Google Scholar]
- Liu X., Farley J. M. Acetylcholine-induced chloride current oscillations in swine tracheal smooth muscle cells. J Pharmacol Exp Ther. 1996 Jan;276(1):178–186. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Ramagopal M. V., Leighton H. J. Effects of NG-monomethyl-L-arginine on field stimulation-induced decreases in cytosolic Ca2+ levels and relaxation in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Dec 19;174(2-3):297–299. doi: 10.1016/0014-2999(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Wayman C. P., McFadzean I., Gibson A., Tucker J. F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996 Feb;117(3):566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


