Abstract
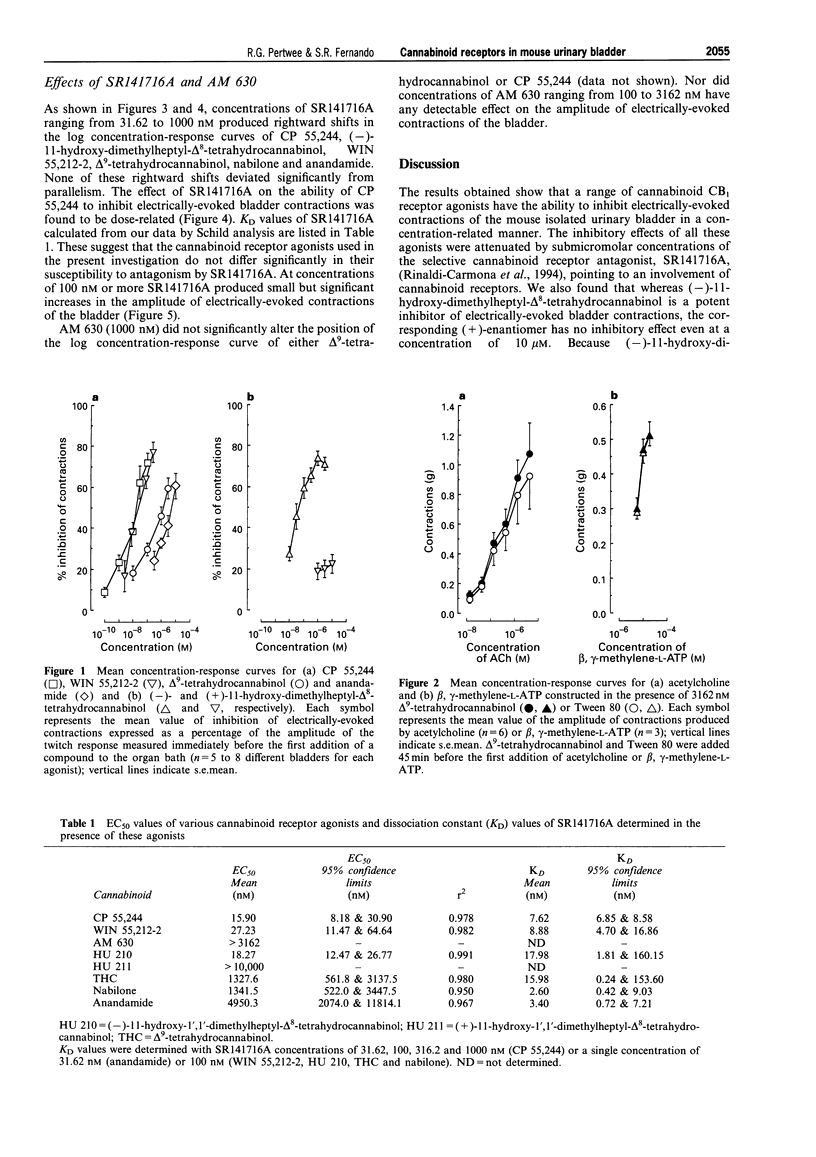
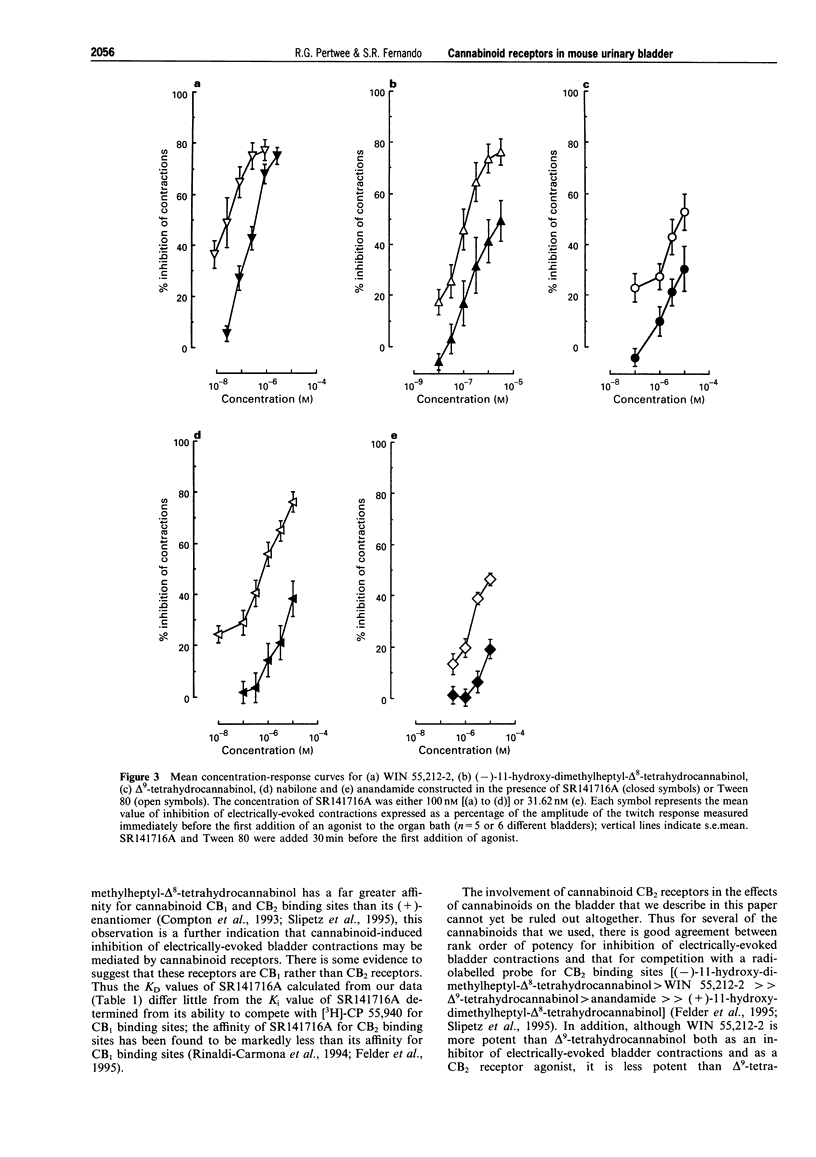
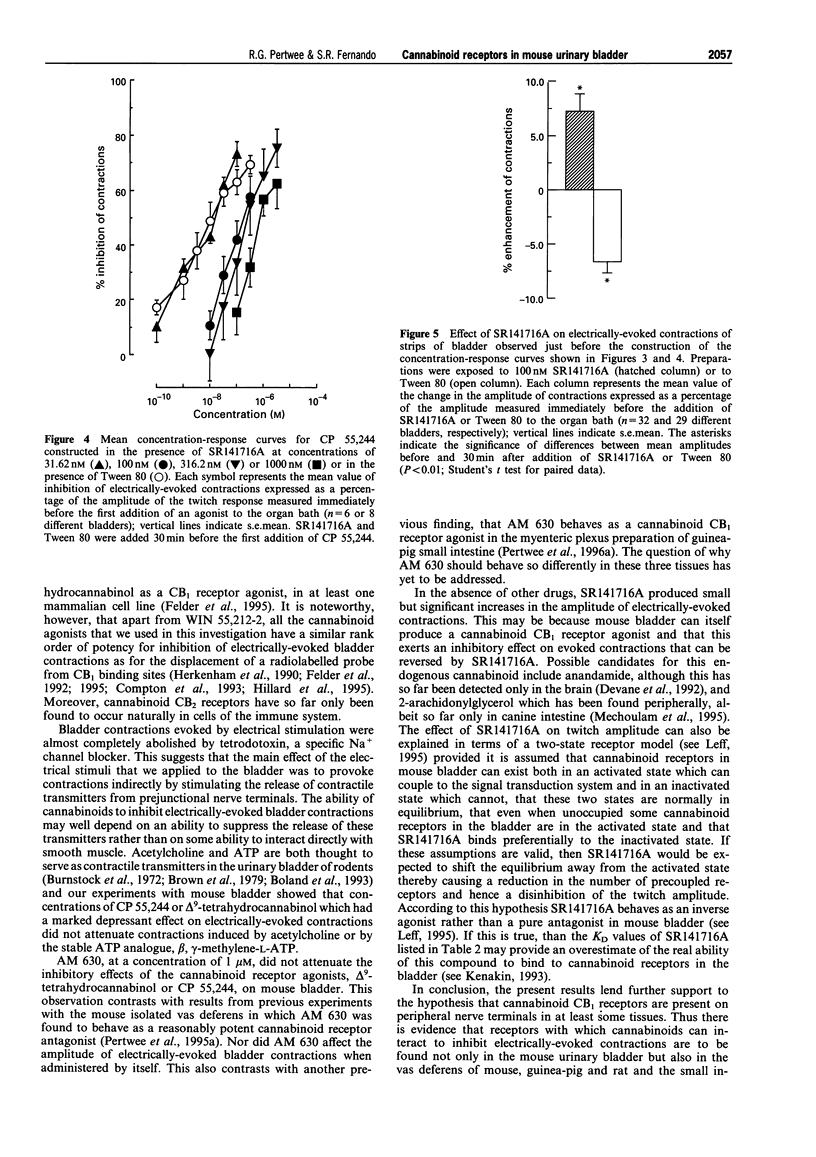
1. CP 55,244, (-)-11-hydroxy-dimethylheptyl-delta 8-tetrahydrocannabinol, WIN 55,212-2, delta 9-tetrahydrocannabinol, nabilone and anandamide each inhibited electrically-evoked contractions of the mouse isolated urinary bladder in a concentration-related manner, their EC50 values being respectively 15.9, 18.27, 27.23, 1327.6, 1341.5 and 4950.3 nM. (+)-11-hydroxy-dimethylheptyl-delta 8-tetrahydrocannabinol was inactive at the highest concentration used (10 microM). 2. SR141716A (31.62 or 100 nM) produced parallel rightward shifts in the log concentration-response curves of CP 55,244, (-)-11-hydroxy-dimethylheptyl-delta 8-tetrahydrocannabinol, WIN 55,212-2, delta 9-tetrahydrocannabinol and anandamide for inhibition of electrically-evoked bladder contractions. The effect of the antagonist on the log concentration-response curve of CP 55,244 was shown to depend on the concentration of SR141716A used (31.62 to 1000 nM). 3. The amplitudes of contractions evoked by acetylcholine or beta, gamma-methylene-L-ATP were not decreased by 316.2 nM CP 55,244 or 3162 nM delta 9-tetrahydrocannabinol. Electrically-evoked contractions were almost completely abolished by 200 nM tetrodotoxin. 4. The above results support the hypothesis that mouse urinary bladder contains prejunctional CB1 cannabinoid receptors which can mediate inhibition of electrically-evoked contractions, probably by reducing contractile transmitter release. 5. AM 630 which behaves as a cannabinoid receptor antagonist in the mouse isolated vas deferens did not antagonize the ability of CP 55,244 or delta 9-tetrahydrocannabinol to inhibit electrically-evoked contractions of the mouse bladder. 6. SR141716A produced small but significant increases in the amplitude of electrically-evoked contractions of the bladder suggesting that this tissue may release an endogenous cannabinoid receptor agonist or that some cannabinoid receptors in this tissue are precoupled to the effector system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland B., Himpens B., Paques C., Casteels R., Gillis J. M. ATP induced-relaxation in the mouse bladder smooth muscle. Br J Pharmacol. 1993 Mar;108(3):749–753. doi: 10.1111/j.1476-5381.1993.tb12872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Burnstock G., Cocks T. Effects of adenosine 5'-triphosphate (ATP) and beta-gamma-methylene ATP on the rat urinary bladder. Br J Pharmacol. 1979 Jan;65(1):97–102. doi: 10.1111/j.1476-5381.1979.tb17337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton D. R., Rice K. C., De Costa B. R., Razdan R. K., Melvin L. S., Johnson M. R., Martin B. R. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993 Apr;265(1):218–226. [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Joyce K. E., Briley E. M., Mansouri J., Mackie K., Blond O., Lai Y., Ma A. L., Mitchell R. L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995 Sep;48(3):443–450. [PubMed] [Google Scholar]
- Felder C. C., Veluz J. S., Williams H. L., Briley E. M., Matsuda L. A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992 Nov;42(5):838–845. [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., de Costa B. R., Richfield E. K. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991 May 3;547(2):267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hillard C. J., Edgemond W. S., Campbell W. B. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995 Feb;64(2):677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995 Mar;16(3):89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Martyn C. N., Illis L. S., Thom J. Nabilone in the treatment of multiple sclerosis. Lancet. 1995 Mar 4;345(8949):579–579. doi: 10.1016/s0140-6736(95)90485-9. [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995 Jun 29;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Pacheco M., Childers S. R., Arnold R., Casiano F., Ward S. J. Aminoalkylindoles: actions on specific G-protein-linked receptors. J Pharmacol Exp Ther. 1991 Apr;257(1):170–183. [PubMed] [Google Scholar]
- Pertwee R. G., Griffin G. A preliminary investigation of the mechanisms underlying cannabinoid tolerance in the mouse vas deferens. Eur J Pharmacol. 1995 Jan 5;272(1):67–72. doi: 10.1016/0014-2999(94)00617-g. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Griffin G., Lainton J. A., Huffman J. W. Pharmacological characterization of three novel cannabinoid receptor agonists in the mouse isolated vas deferens. Eur J Pharmacol. 1995 Sep 25;284(3):241–247. doi: 10.1016/0014-2999(95)00318-f. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Joe-Adigwe G., Hawksworth G. M. Further evidence for the presence of cannabinoid CB1 receptors in mouse vas deferens. Eur J Pharmacol. 1996 Jan 25;296(2):169–172. doi: 10.1016/0014-2999(95)00790-3. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Stevenson L. A., Elrick D. B., Mechoulam R., Corbett A. D. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol. 1992 Apr;105(4):980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R., Griffin G., Fernando S., Li X., Hill A., Makriyannis A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56(23-24):1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M., Barth F., Héaulme M., Shire D., Calandra B., Congy C., Martinez S., Maruani J., Néliat G., Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994 Aug 22;350(2-3):240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Slipetz D. M., O'Neill G. P., Favreau L., Dufresne C., Gallant M., Gareau Y., Guay D., Labelle M., Metters K. M. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol Pharmacol. 1995 Aug;48(2):352–361. [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]


